Almotriptan
Bezeichnung:Almotriptan
CAS-Nr154323-57-6
Englisch Name:Almotriptan
CBNumberCB7716603
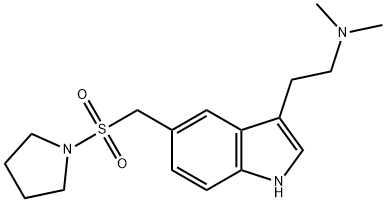
SummenformelC17H25N3O2S
Molgewicht335.46
MOL-Datei154323-57-6.mol
Almotriptan physikalisch-chemischer Eigenschaften
| Siedepunkt | 538.7±60.0 °C(Predicted) |
| Dichte | 1.27±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| Aggregatzustand | Solid |
| pka | 16.92±0.30(Predicted) |
| Farbe | White to off-white |
| CAS Datenbank | 154323-57-6(CAS DataBase Reference) |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)

-
Alarmwort
Warnung
-
Gefahrenhinweise
H315:Verursacht Hautreizungen.
H319:Verursacht schwere Augenreizung.
H335:Kann die Atemwege reizen.
-
Sicherheit
P233:Behälter dicht verschlossen halten.
P260:Dampf/Aerosol/Nebel nicht einatmen.
P261:Einatmen von Staub vermeiden.
P264:Nach Gebrauch gründlich waschen.
P264:Nach Gebrauch gründlich waschen.
P270:Bei Gebrauch nicht essen, trinken oder rauchen.
P271:Nur im Freien oder in gut belüfteten Räumen verwenden.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P301+P312:BEI VERSCHLUCKEN: Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P302+P352:BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen.
P304:BEI EINATMEN
P304+P340:BEI EINATMEN: Die Person an die frische Luft bringen und für ungehinderte Atmung sorgen.
P305+P351+P338:BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen.
P312:Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P321:Besondere Behandlung
P330:Mund ausspülen.
P332+P313:Bei Hautreizung: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
P337+P313:Bei anhaltender Augenreizung: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
P340:Die betroffene Person an die frische Luft bringen und in einer Position ruhigstellen, die das Atmen erleichtert.
P362:Kontaminierte Kleidung ausziehen und vor erneutem Tragen waschen.
P403:An einem gut belüfteten Ort aufbewahren.
P403+P233:An einem gut belüfteten Ort aufbewahren. Behälter dicht verschlossen halten.
P405:Unter Verschluss aufbewahren.
P501:Inhalt/Behälter ... (Entsorgungsvorschriften vom Hersteller anzugeben) zuführen.
Almotriptan Chemische Eigenschaften,Einsatz,Produktion Methoden
-
Beschreibung
Almotriptan was first marketed in Spain as a new medicine against acute attacks of migraine. It is the fifth agent belonging to the “triptan” class to be launched after sumatriptan, naratriptan, zolmitriptan and rizatriptan. This close structural analog of sumatriptan can be prepared in six steps from 4-nitrobenzylsulfonyl chloride with a Fischer indole synthesis as the key step. Almotriptan acts as a dual 5-HT1D/1B agonist with a 35 to 51-fold selectivity versus 5-HT1A and 5-HT7 receptors respectively as well as having insignificant affinity for the most relevant nonserotonergic receptors (K1>1μM). Its agonistic effect on 5-HT,n receptors of trigeminal sensory neurons turns off neurogenic inflammation by inhibiting the release of neuropeptides such as calcitonin gene-related peptide, neurokinin A and substance P. Concomitantly, its action on the 5-HT1B receptors in meningeal arteries relieves the vasodilatation of these vessels associated with migraine attacks. Almotriptan causes selective concentration-dependent vasoconstriction of human meningeal and temporal arteries (with EC50 of 0.03 and 0.7 μM) compared to basilar (EC50 = 3.5 μM) and pulmonary arteries (EC50>10μM) or rabbit mesenteric and renal arteries (EC50>100 μM). Although it is predominantly cleared by the kidneys as unchanged drug (45%) or transformed into inactive metabolites by monoamine oxidase A (MAO-A) and CYP3A4 enzymes in the liver, almotriptan has the highest oral bioavailability (70%) of the triptans and has a half-life of 3.5 h. The therapeutic dose of 12.5 mg is well tolerated, shows a rapid onset of action (30 min) and low recurrence rate compared to sumatriptan. -
Originator
Almirall Prodesfarma (Spain) -
Verwenden
Serotonin 5HT1B /1D-receptor agonist -
Definition
ChEBI: An indole compound having a 2-(dimethylamino)ethyl group at the 3-position and a (pyrrolidin-1-ylsulfonyl)methyl group at the 5-position. -
Manufacturing Process
To a solution of previously dried 1-[[2-carboxy-3-(2-dimethylaminoethyl)-5- indolyl]methanesulphonyl]-pyrrolidine (1.6 g; 0.0442 moles) in anhydrous quinoline (75 ml) and under atmosphere of nitrogen, cuprous oxide (160 mg; 0.0011 moles) was added. The reaction mixture was heated to 190°C for 15 minutes, stirred to room temperature, poured into a mixture of 1 N hydrochloric acid (150 ml) and ethyl acetate (50 ml), shaken and decanted. The aqueous solution was washed several times with ethyl acetate, then solid sodium bicarbonate was added until pH = 7.8, and washed with n-hexane to eliminate the quinoline. The aqueous solution was made alkaline with solid potassium carbonate and extracted with ethyl acetate. The organic solution was dried (Na2SO4), the solvent removed under reduced pressure when a dark oil was obtained (1.3 g; yield 92%). This product was purified by column chromatography with silica gel and methylene chloride:ethanol:ammonium hydroxide (60:8:1) as eluent and a white foam (0.8 g) of 1-[[3-(2- dimethylaminoethyl)-5-indolyl]methanesulphonyl]-pyrrolidine was obtained. To a solution of the above product (0.8 g) in acetone (30 ml), a few drops of hydrogen chloride saturated dioxan solution, were added. The precipitated solid was collected by filtration, washed with acetone and dried to give 1-[(3- (2-(dimethylamino)ethyl)-5-indolyl)methanesulphonyl]-pyrrolidine hydrochloride (0.75 g). Melting point 218°-220°C.
In practice it is usually used as malate salt. -
Trademarks
Almogran -
Therapeutic Function
Migraine therapy -
Clinical Use
5HT1 receptor agonist:
Acute relief of migraine -
Arzneimittelwechselwirkung
Potentially hazardous interactions with other drugs
Antidepressants: increased risk of CNS toxicity with citalopram - avoid; possibly increased serotonergic effects with duloxetine or venlafaxine; increased serotonergic effects with St John’s wort - avoid.
Antifungals: concentration increased by ketoconazole (increased risk of toxicity).
Dapoxetine: possible increased risk of serotonergic effects - avoid for 2 weeks after stopping 5HT1 agonists.
Ergot alkaloids: increased risk of vasospasm - avoid. -
Stoffwechsel
The major biotransformation route is via monoamine oxidase (MAO-A) mediated oxidative deamination to the indole acetic metabolite. Cytochrome P450 (3A4 and 2D6 isozymes) and flavin mono-oxygenase are other enzymes involved in the metabolism of almotriptan. None of the metabolites are significantly active pharmacologically. More than 75% of a dose is eliminated in urine, and the remainder in faeces. Approximately, 50% of the urinary and faecal excretion is unchanged almotriptan.
Almotriptan Upstream-Materialien And Downstream Produkte
Upstream-Materialien
- Kupferoxid
- Chinolin
- Pyrrolidin
- 1H-Indole-3-ethanol, 5-[(1-pyrrolidinylsulfonyl)methyl]-
- Butanal, 4-(dimethylamino)-, 2-[4-[(1-pyrrolidinylsulfonyl)methyl]phenyl]hydrazone
1of3
Almotriptan Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(115)Suppliers
-
Telefon +undefined-21-51877795
E-Mail ivan@atkchemical.com
-
Telefon +86-0371-86658258<br/>+8613203830695
E-Mail sales@coreychem.com
-
Telefon +86-86-5926051114<br/>+8618959220845
E-Mail sales@amoychem.com
-
Telefon +86-023-6139-8061<br/>+86-86-13650506873
E-Mail sales@chemdad.com
-
CONIER CHEM AND PHARMA LIMITED
Telefon +8618523575427
E-Mail sales@conier.com
-
Telefon +86-13806087780
E-Mail sale@simagchem.com
-
Telefon +1-781-999-5354<br/>+1-00000000000
E-Mail marketing@targetmol.com
-
Telefon +86-0571-85134551
E-Mail sales@afinechem.com
-
Dayang Chem (Hangzhou) Co.,Ltd.
Telefon 571-88938639<br/>+8617705817739
E-Mail info@dycnchem.com
-
changzhou huayang technology co., ltd
Telefon +8615250961469
E-Mail 2571773637@qq.com
1of2
