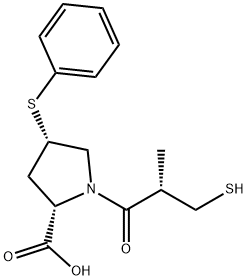
Zofenoprilat
Bezeichnung:Zofenoprilat
CAS-Nr75176-37-3
Englisch Name:Zofenoprilat
CBNumberCB6162467
SummenformelC15H19NO3S2
Molgewicht325.45
MOL-Datei75176-37-3.mol
Zofenoprilat physikalisch-chemischer Eigenschaften
| Siedepunkt | 556.2±50.0 °C(Predicted) |
| Dichte | 1.31±0.1 g/cm3(Predicted) |
| pka | 3.32±0.40(Predicted) |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)

-
Alarmwort
Achtung
-
Gefahrenhinweise
H301:Giftig bei Verschlucken.
H311:Giftig bei Hautkontakt.
H331:Giftig bei Einatmen.
-
Sicherheit
P261:Einatmen von Staub vermeiden.
P264:Nach Gebrauch gründlich waschen.
P264:Nach Gebrauch gründlich waschen.
P270:Bei Gebrauch nicht essen, trinken oder rauchen.
P271:Nur im Freien oder in gut belüfteten Räumen verwenden.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P302+P352:BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen.
P304+P340:BEI EINATMEN: Die Person an die frische Luft bringen und für ungehinderte Atmung sorgen.
P310:Sofort GIFTINFORMATIONSZENTRUM/Arzt/ anrufen.
P330:Mund ausspülen.
P361:Alle kontaminierten Kleidungsstücke sofort ausziehen.
P403+P233:An einem gut belüfteten Ort aufbewahren. Behälter dicht verschlossen halten.
P405:Unter Verschluss aufbewahren.
P501:Inhalt/Behälter ... (Entsorgungsvorschriften vom Hersteller anzugeben) zuführen.
Zofenoprilat Chemische Eigenschaften,Einsatz,Produktion Methoden
-
Chemische Eigenschaften
White crystalline -
Originator
Zofenil arginine,Menarini -
Verwenden
The active metabolite of Zofenopril, an ACE inhibitor -
Definition
ChEBI: A proline derivative that is 4-(phenylsulfanyl)-L-proline in which the amine proton is replaced by a (2S)-2-methyl-3-sulfanylpropanoyl group. The active metabolite of zofenopril. -
Manufacturing Process
Sodium metal (0.85 g, 0.037 mole) is dissolved in 40 ml of absolute ethanol. To this there is added with stirring 3.7 ml (0.036 mole) of thiophenol followed by 7.5 g (0.017 mole) of N-carbobenzyloxy-trans-4-tosyloxy-L-proline, methyl ester [J. Am. Chem. Soc., 79, 191 (1957)]. After stirring for 4 h and standing overnight at room temperature, the bulk of the ethanol is removed on a rotary evaporator. The mostly solid residue is stirred with 120 ml of dichloromethane and 60 ml of water. The layers are separated (some methanol is added to help break up emulsions) and the aqueous phase is extracted with additional dichloromethane (2x60 ml). The combined organic phase are washed with 100 ml of saturated sodium chloride solution, dried (MgSO 4 ), and the solvent evaporated to give 6.5 g (100%) of N-carbobenzyloxy-cis-4-phenylthio-L- proline, methyl ester as a pale yellow viscous oil.
The N-carbobenzyloxy-cis-4-phenylthio-L-proline, methyl ester (6.5 g, 0.017 mole) is dissolved in 55 ml of methanol, treated portionwise at -1° to 4°C with 13 ml (0.026 mole) of 2 N sodium hydroxide, stirred at 0°C for 1 h, and kept at room temperature for approximately 16 h. After removing about half of the solvent on a rotary evaporator, the cooled solution is diluted with 100 ml of water, washed with 60 ml of ether (wash discarded), layered over with 70 ml of ethyl acetate, stirred, cooled, and acidified with 4.8 ml of 1:1 hydrochloric acid. After separating, the aqueous phase is extracted with additional ethyl acetate (3x40 ml) and the combined organic layers are dried (MgSO 4 ) and evaporated to give 5.9 g of a light yellow viscous oil. The latter is dissolved in 30 ml of ethanol, treated with 1.9 g of cyclohexylamine in 3 ml of ethanol and diluted to 330 ml with ether. On seeding, the crystalline cyclohexylamine salt separates. The latter, after cooling for approximately 16 h, weighs 5.3 g; melting point 148-151°C. This material is combined with 1.5 g of identical product from a previous experiment, stirred with 200 ml of boiling acetonitrile, and cooled to yield 6.3 g of colorless N-carbobenzyloxy- cis-4-phenylthio-L-proline cyclohexylamine salt; melting point 152-155°C.
This N-carbobenzyloxy-cis-4-phenylthio-L-proline cyclohexylamine salt is suspended in 25 ml of ethyl acetate, stirred, and treated with 25 ml of 1 N hydrochloric acid. When two clear layers are obtained, they are separated and the aqueous phase is extracted with additional ethyl acetate (3x25 ml). The combined organic layers are dried (MgSO 4 ) and the solvent evaporated to give 5.0 g (65%) of N-carbobenzyloxy-cis-4-phenylthio-L-proline as a nearly colorless, very viscous syrup.
N-Carbobenzyloxy-cis-4-phenylthio-L-proline (4.9 g, 0.014 mole) is treated with 25 ml of hydrogen bromide in acetic acid (30-32%), stoppered loosely, and stirred magnetically. After 1 h the orange-yellow solution is diluted to 250 ml with ether to precipitate the product as a heavy oil which gradually crystallizes on seeding, rubbing and cooling After stirring in an ice-bath for 1 h, the material is filtered under nitrogen, washed with ether, suspended in fresh ether, cooled for approximately 16 h, and filtered again to give 3.2 g (77%) of colorless solid (cis)-4-phenylthio-L-proline hydrobromide; melting point 106-109°C.
A solution of 3.0 g (0.0094 mole) of (cis)-4-phenylthio-L-proline hydrobromide in 25 ml of water is stirred, cooled to 5°C and 15 ml of 20% sodium carbonate are added. This mixture is treated with 2.0 g (0.011 mole) of D-3- acetylthio-2-methylpropionyl chloride in 5 ml of ether during the course of 10 min with the intermittent addition of 3.0 g of sodium carbonate to maintain the pH at 8.0 to 8.4). The mixture is stirred in the ice-bath for an additional hour, 25 ml of water are added and then a solution of 5 ml of concentrated hydrochloric acid in 25 ml of water. The strongly acid solution is saturated with sodium chloride and extracted with 50 ml of ethyl acetate (four ti -
Therapeutic Function
Antihypertensive
Zofenoprilat Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(61)Suppliers
-
Nanjing ChemLin Chemical Industry Co., Ltd.
Telefon 025-83697070
E-Mail product@chemlin.com.cn
-
Hubei Jusheng Technology Co.,Ltd.
Telefon 18871490254
E-Mail linda@hubeijusheng.com
-
CONIER CHEM AND PHARMA LIMITED
Telefon +8618523575427
E-Mail sales@conier.com
-
Telefon +86-0371-86658258<br/>+8613203830695
E-Mail factory@coreychem.com
-
Telefon +86-13806087780
E-Mail sale@simagchem.com
-
Telefon +86-0571-85134551
E-Mail sales@afinechem.com
-
Telefon +86-0571-81612335<br/>+8613336028439
E-Mail sales@nextpeptide.com
-
LUYUNJIA CHEMISTRY XIAMEN LIMITED
Telefon +86-592-5360779<br/>+86-13055435203
E-Mail
-
Telefon +86-852-30606658
E-Mail market18@leapchem.com
-
Xiamen Eagle Chemical Limited Corporation
Telefon
E-Mail yiyunchem@163.com
1of2
