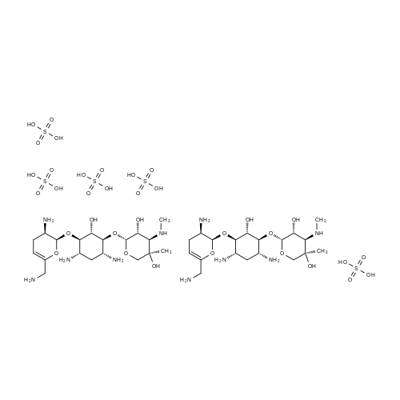
Disisomicinpentakis(sulfat)
Bezeichnung:Disisomicinpentakis(sulfat)
CAS-Nr53179-09-2
Englisch Name:Sisomycin Sulfate
CBNumberCB5402393
SummenformelC19H39N5O11S
Molgewicht545.61
MOL-Datei53179-09-2.mol
Synonyma
Disisomicinpentakis(sulfat)
Disisomicinpentakis(sulfat) physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 70-73C |
| Siedepunkt | 155℃ |
| Flammpunkt | >110°(230°F) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| Löslichkeit | H2O: soluble50mg/mL |
| Aggregatzustand | powder |
| Farbe | white to off-white |
| Optische Aktivität | [α]25/D 100 to 110° in water (Specific rotation (anhydrous), C=1) |
| Wasserlöslichkeit | Soluble in water |
| Merck | 14,8550 |
| Stabilität | Hygroscopic |
| Kennzeichnung gefährlicher | T,Xi |
| R-Sätze: | 61-20/21/22-36/37/38 |
| S-Sätze: | 53-22-36/37/39-45-36-26 |
| WGK Germany | 3 |
| RTECS-Nr. | WK2288000 |
| HS Code | 2941901010 |
| Toxizität | LD50 in mice (mg/kg): 34 i.v., 221 i.p., 288 s.c. (Weinstein) |


