Calcitonin
Bezeichnung:Calcitonin
CAS-Nr9007-12-9
Englisch Name:Calcitonin
CBNumberCB5127317
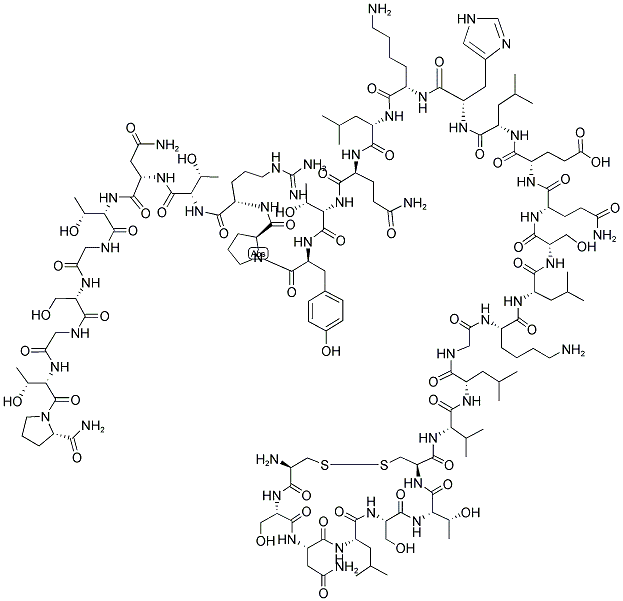
SummenformelC145H240N44O48S2
Molgewicht3431.85
MOL-Datei9007-12-9.mol
Synonyma
Calcitonin
Calcitonin physikalisch-chemischer Eigenschaften
| storage temp. | −20°C |
| Löslichkeit | 0.05 M acetic acid: 1 mg/mL, clear, colorless |
| Aggregatzustand | powder |
| S-Sätze: | 22-24/25 |
| WGK Germany | 3 |
| RTECS-Nr. | EV8000000 |
| F | 3-10 |
| Giftige Stoffe Daten | 9007-12-9(Hazardous Substances Data) |
Calcitonin Chemische Eigenschaften,Einsatz,Produktion Methoden
-
S-Sätze Betriebsanweisung:
S22:Staub nicht einatmen.
S24/25:Berührung mit den Augen und der Haut vermeiden. -
Beschreibung
Calcitonin has been approved for the treatment of postmenopausal osteoporosis, hypercalcemia of malignancy, and Paget's disease of the bone.Several sources are available (e.g., eel, human, salmon, and porcine). The calcitonin isolated from salmon is the preferred source, because it has greater receptor affinity and a longer half-life than the human hormone. -
Chemische Eigenschaften
Calcitonin is a single-chain polypeptide composed of 32 amino acid residues having a molecular weight of approximately 3600. A cysteine disulfide bridge at the 1-7 position of the amino terminal end of the peptide is essential for biological activity; however, the entire amino acid sequence is required for optimal activity. -
Originator
Calcitar,Yamanouchi,Japan,1978 -
Verwenden
Regulator (calcium). -
Indications
Calcitonin (Miacalcin, Miacalcin Nasal Spray) is a synthetic 32–amino acid polypeptide that is identical to salmon calcitonin. Salmon calcitonin is more potent than human calcitonin because of its higher affinity for the human calcitonin receptor and its slower metabolic clearance. Administration is by subcutaneous or intramuscular injection or by nasal spray. The absorption of the nasal form is slower than that of the parenteral routes. -
Indications
Calcitonin release is normally stimulated by rising serum calcium levels and suppressed by hypocalcemia. The major physiological effects of calcitonin are inhibition of bone resorption and deposition of postabsorptive calcium into bone following a meal, which prevents postprandial hypercalcemia. -
Manufacturing Process
The process for the manufacture of human calcitonin in pure form from C-cell rich medulla carcinoma of the thyroid gland or from C-cell metastasis material is one wherein medullar carcinoma of the thyroid gland or C-cell metastasis material, which has been defatted, for example with acetone or ether, and which may have been first purified with alcohol or with aqueous trichloroacetic acid, is extracted one or more times with a solvent system containing water and an alkanol having at most 5 carbon atoms, at a pH of from about 1 to 6, and the extracted product subjected to gel chromatography using aqueous formic acid as eluant. The calcitonin may be separated into its constituents by countercurrent distribution, for example by Craig distribution using a solvent system advantageously containing n-butanol and acetic acid. -
Trademarks
Calcimar (Rhone- Poulenc Rorer);. -
Therapeutic Function
Calcium regulator -
Biosynthese
The regulation of calcitonin synthesis and release from the parafollicular C cells of the thyroid gland is calcium dependent. Rising serum calcium is the principal stimulus responsible for calcitonin synthesis and release. Other hormones, such as glucagon, gastrin, and serotonin, also stimulate calcitonin release. Calcitonin has been isolated in tissues other than the parafollicular C cells (parathyroid, pancreas, thymus, adrenal), but it is not known whether this material is biologically active.
Secretagogues, such as gastrin and pancreozymin, may contribute significantly to the regulation of endogenous calcitonin. In fact, it has been postulated that gastrin-induced calcitonin release following meals may help regulate the postprandial calcium deposition in bone.
A calcitonin precursor has been identified within the thyroid parafollicular C cells. It is thought to function in a manner analogous to that of proPTH to facilitate intracellular transport and secretion of the hormone. The metabolic degradation of calcitonin appears to occur in both the liver and kidney.
Although blood calcitonin levels are normally low, excessive levels have been found in association with medullary carcinoma of the thyroid and more rarely carcinoid tumors of the bronchus and stomach. Serum calcitonin levels are used to screen and monitor patients who have or are suspected of having medullary carcinoma of the thyroid. -
Biologische Funktion
In addition to its antiresorptive action via suppression of osteoclast activity, calcitonin-salmon exhibits a potent analgesic effect and has provided considerable relief to those patients suffering from the pain associated with Paget's disease and osteoporosis. This analgesic effect is a result of calcitonin-stimulated endogenous opioid release. The potency of this analgesic effect has been demonstrated to be 30- to 50-fold that of morphine in selected patients. Calcitonin is preferred over estrogen and the bisphosphonates when treatment of both osteoporosis and related bone pain is warranted. -
Acquired resistance
Resistance to calcitonin-salmon can result from the development of neutralizing antibodies. -
Allgemeine Beschreibung
Calcitonin (thyrocalcitonin) is a 32-amino-acid polypeptidehormone secreted by parafollicular cells of the thyroidglands in response to hypocalcemia. The entire 32-residuepeptide appears to be required for activity, because smallerfragments are totally inactive. Common structural featuresof calcitonin isolated from different species are a COOHterminalprolinamide, a disulfide bond between residues 1and 7 at the NH2 terminus, and a chain length of 32 residues.Calcitonin inhibits calcium resorption from bone, causinghypocalcemia, with parallel changes in plasma phosphateconcentration. In general, calcitonin negates the osteolyticeffects of parathyroid hormone.
The potential therapeutic uses of calcitonin are in thetreatment of hyperparathyroidism, osteoporosis and otherbone disorders, hypercalcemia of malignancy, and idiopathichypercalcemia. -
Mechanism of action
Calcitonin interacts with specific plasma membrane receptors within target organs to initiate biological effects. This interaction has been directly linked to the generation of cAMP via adenylyl cyclase activation. -
Pharmakokinetik
Calcitonin-salmon differs structurally from human calcitonin at 16 of 32 amino acids. The pharmacological activity of these calcitonins is the same, but calcitonin-salmon is approximately 50-fold more potent on a weight basis than human calcitonin with a longer duration of action. The duration of action for calcitonin salmon is 8 to 24 hours following intramuscular (IM) or subcutaneous (SC) administration and 0.5 to 12.0 hours following IV administration. The parenteral dose required for the treatment of osteoporosis is 100 IU/day. Initially only available by IM or SC injection, the peptide hormone calcitonin-salmon is available as a nasal spray (Miacalcin) and as a rectal suppository. A recombinant DNA form of calcitonin salmon was approved by the U.S. FDA in 2005 and is available as a nasal spray. The bioavailability of calcitonin-salmon nasal spray shows great variability (range, 0.3–30.6% of an IM dose). It is absorbed rapidly from the nasal mucosa, with peak plasma concentrations appearing 30 to 40 minutes after nasal administration, compared with 16 to 25 minutes following parental dosing. Calcitonin-salmon is readily metabolized in the kidney, with an elimination half-life calculated at 43 minutes. As a result, the intranasal dose required is 200 IU/day. Once the Miacalcin nasal pump has been activated, the bottle may be kept at room temperature until the medication is finished (2 weeks) -
Clinical Use
Calcitonin therapy requires the concomitant oral administration of elemental calcium (500 mg/day). Clinical studies have shown that the combination of intranasal calcitonin salmon (200 IU/day), oral calcium supplementation (>1,000 mg/day of elemental calcium), and vitamin D (400 IU/day) has decreased the rate of new fractures by more than 75% and has improved vertebral BMD by as much as 3% annually. Calcitonin prevents the abnormal bone turnover characteristic of Paget's disease of the bone and has antiresorptive activity. In the presence of calcitonin, the osteoclast brush borders disappear, and the osteoclasts move away from the bone surface undergoing remodeling. Side effects are significantly more pronounced when calcitonin-salmon is administered by injection and can include nausea, vomiting, anorexia, and flushing. Because calcitonin-salmon is protein in nature, the possibility of a systemic allergic reaction should be considered,and appropriate measures for treatment of hypersensitivity reaction should be readily available. Although calcitonin-salmon does not cross the placenta, it may pass into breast milk. Calcitonin-salmon is a possible alternative to ERT; however, only limited evidence suggests that it has efficacy in women who already have fractures.
Calcitonin Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(64)Suppliers
-
Nantong Guangyuan Chemicl Co,Ltd
Telefon +undefined17712220823
E-Mail admin@guyunchem.com
-
Henan Tianfu Chemical Co.,Ltd.
Telefon +86-0371-55170693<br/>+86-19937530512
E-Mail info@tianfuchem.com
-
Telefon 029-89275612<br/>+8618991951683
E-Mail mcbio_sales@163.com
-
Dideu Industries Group Limited
Telefon +86-29-89586680<br/>+86-15129568250
E-Mail 1026@dideu.com
-
Telefon
E-Mail support@targetmol.com
-
Changyi Longchang Bio-Chemical Co., LTD
Telefon +8613256193735
E-Mail info@longchangchemical.com
-
Telefon +8617320513646
E-Mail mike@molcoo.com
-
Telefon 86-21-51086038
E-Mail chemwill_asia@126.com
-
Telefon 18059081430
E-Mail 2070812664@qq.com
-
3B Pharmachem (Wuhan) International Co.,Ltd.
Telefon 821-50328103-801<br/>18930552037
E-Mail 3bsc@sina.com
1of2
