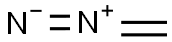
Diazomethan
Bezeichnung:Diazomethan
CAS-Nr334-88-3
Englisch Name:Diazomethane
CBNumberCB4852342
SummenformelCH2N2
Molgewicht42.03998
MOL-Datei334-88-3.mol
Synonyma
Diazomethan
Azimethylen
Diazirine
Diazomethan physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -145° |
| Siedepunkt | bp -23° |
| Dichte | 1.45 g/cm3 |
| Brechungsindex | 1.4180 (estimate) |
| Aggregatzustand | Yellow gas |
| Geruch (Odor) | Musty odor (no accepted threshold value) |
| Expositionsgrenzwerte | TLV-TWA 0.2 ppm (0.38 mg/m3 ) (ACGIH) PEL-TWA 0.2 ppm (0.38 mg/m3 ) (OSHA). |
| IARC | 3 (Vol. 7, Sup 7) 1987 |
| EPA chemische Informationen | Diazomethane (334-88-3) |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 45 |
| S-Sätze: | 53-45 |
| RIDADR | 1953 |
| OEB | C |
| OEL | TWA: 0.2 ppm (0.4 mg/m3) |
| Selbstentzündungstemperatur | 150 °C; impure material explodes at lower temperature |
| HazardClass | 2.3 |
| Giftige Stoffe Daten | 334-88-3(Hazardous Substances Data) |
| Toxizität | LCLO inhal (cat) 175 ppm (10 min) PEL (OSHA) 0.2 ppm (0.4 mg/m3) TLV-TWA (ACGIH) 0.2 ppm (0.4 mg/m3) |
| IDLA | 2 ppm |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)

-
Alarmwort
Achtung
-
Gefahrenhinweise
H350:Kann Krebs verursachen.
Diazomethane Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
GELBES GAS. -
PHYSIKALISCHE GEFAHREN
Das Gas ist schwerer als Luft und kann sich am Boden ausbreiten. Fernzündung möglich. -
CHEMISCHE GEFAHREN
Bei Stoß, Reibung oder Erschütterung explosionsartige Zersetzung möglich. Kann beim Erhitzen auf 100°C, bei Kontakt mit rauhen Oberflächen, wenn Verunreinigungen oder Feststoffe in der unverdünnten Flüssigkeit oder in konzentrierten Lösungen vorhanden sind oder unter starkem Lichteinfluss explodieren. Kontakt mit Alkalimetallen und Calciumsulfat verursacht Explosionen. -
ARBEITSPLATZGRENZWERTE
TLV: 0,2 ppm (als TWA); Krebskategorie A2 (Verdacht auf krebserzeugende Wirkung beim Menschen); (ACGIH 2008).
MAK: Krebserzeugend Kategorie 2; (DFG 2008).
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation. -
INHALATIONSGEFAHREN
Eine gesundheitsschädliche Konzentration des Gases in der Luft wird beim Entweichen aus dem Behälter sehr schnell erreicht. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz verätzt stark die Augen, die Haut und die Atemwege. Inhalation des Dampfes kann zu Lungenödem und Asthma führen (s.Anm.). Die Flüssigkeit kann Erfrierungen hervorrufen. Exposition oberhalb der Arbeitsplatzgrenzwerte kann zum Tod führen. ärztliche Beobachtung notwendig. -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholte oder andauernde Inhalation kann asthmatische Beschwerden hervorrufen. Möglicherweise krebserzeugend für den Menschen. -
LECKAGE
Gefahrenbereich verlassen! Fachmann zu Rate ziehen! Belüftung. Persönliche Schutzausrüstung: Vollschutzanzug mit umgebungsluftunabhängigem Atemschutzgerät. -
R-Sätze Betriebsanweisung:
R45:Kann Krebs erzeugen. -
S-Sätze Betriebsanweisung:
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen). -
Aussehen Eigenschaften
CH2N2. Dunkelgelbe, bei 23oC siedende Flüssigkeit -> gelbes, nach feuchtem Laub riechendes, sehr giftiges Gas. Löslich in Ether. -
Gefahren für Mensch und Umwelt
Diazomethan ist sehr explosiv. Bereits das Vorhandensein scharfer Kanten in der Apparatur kann eine Explosion initiieren. Über 100oC thermische Zersetzung.
Diazomethan ist sehr giftig. Es greift Haut, Lunge und Augen an und ruft asthmatische Beschwerden hervor.
Kann Krebs erzeugen. -
Schutzmaßnahmen und Verhaltensregeln
Nur im Abzug arbeiten! Möglichst nicht mit dem reinen Gas arbeiten (besser mit einer Lösung von Diazomethan z.B. in Ether). Schutzschilde (Splitterschutz) um die Apparatur aufstellen.
Schutzhandschuhe (nur als kurzzeitiger Spritzschutz).
Jeden direkten Kontakt mit Diazomethan vermeiden. -
Verhalten im Gefahrfall
Bei Gasaustritt maximale Lüftungsstufe einstellen. Labor räumen. Zündquellen fernhalten.
Im Brandfall Sicherheitszone bilden. Explosionsgefahr! -
Erste Hilfe
Nach Hautkontakt: Betroffene Körperstellen mit Wasser spülen.
Nach Augenkontakt: Mit Wasser mindestens 15 Minuten bei geöffnetem Augenlid spülen. Augenarzt!
Nach Einatmen: Frischluft. Arzt!
Nach Kleidungskontakt: Benetzte Kleidung sofort ausziehen.
Ersthelfer: siehe gesonderten Anschlag -
Sachgerechte Entsorgung
Lösung von Diazomethan in etherische Carbonsäurelösung eintropfen (Bildung des Methylesters). Nach der Reaktion (Entfärbung) als Lösemittelabfall entsorgen. -
Beschreibung
Diazomethane is a flammable, yellow gas, or aliquid under pressure. Molecular weight = 42.05; Boilingpoint = 222.7℃; Freezing/Melting point = -145℃;Autoignition temperature = 100℃ (explodes). HazardIdentification (based on NFPA-704 M Rating System):Health 4, Flammability 3, Reactivity 3. Decomposes inwater; reaction. -
Chemische Eigenschaften
Diazomethane is a flammable, yellow gas or a liquid under pressure. Musty odor. -
Verwenden
Powerful methylating agent for acidic compounds such as carboxylic acids, phenols, enols; not manufactured for sale and distribution because of toxicity and explosivity -
Verwenden
It is used in organic synthesis as a methylating agent to methylate acidic compoundssuch as carboxylic acids and phenols. Itis used in trace environmental analysis tomethylate chlorophenoxy acid herbicides. -
Verwenden
Powerful methylating agent for acidic Compounds such as carboxylic acids, phenols, enols. For syntheses with diazomethane see the reviews by Smith, Chem. Rev. 23, 193 (1938); Eistert, Z. Angew. Chem. 54, 99, 124 (1941) translated by Spangler in Newer Methods of Preparative Organic Chemistry (New York, 1948) p 513; J. S. Pizey, Synthetic Reagents vol. 2 (John Wiley, New York, 1974) pp 65-142. -
Vorbereitung Methode
Two main methods are used to prepare diazomethane. One uses commercially available apparatus specifically designed for its preparation and distillation while entrained with ether. The resulting ether solution is typically of 0.3–0.4 m concentration and diazomethane is in its purest form. Such apparatus have specialized joints without ground glass and come in a range of sizes for generating diazomethane on scales of around 1, 50, or 300 mmol. The other method uses conventional glassware. Both methods use hydroxide to generate the diazomethane from nitrosamide precursors. The more formal method involves adding N-methyl-N-nitroso-toluenesulfonamide (Fig. 1), also known as Diazald, to KOH. The manufacturer's instructions for the use of this apparatus should be followed explicitly.
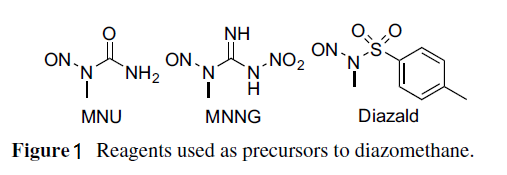
Figure 1 Reagents used as precursors to diazomethane.
The home brew method to make diazomethane can be found in its original form in Organic Syntheses (using N-methyl-N-nitrosourea as the precursor) (De Boer and Backer, 1956). This method uses a two-phase system of 50% aqueous KOH and diethyl ether in an Erlenmeyer flask cooled in an ice-water bath and stirred magnetically. The precursor recommended today, because it is safer to store and handle, is the crystalline solid N-methyl-N-nitroso-nitro-guanidine (MNNG). However, MNNG is still considered toxic, a severe irritant, a carcinogen, and a mutagen, and is typically used for generation of diazomethane quantities of 1 mmol. MNNG is slowly added to the two-phase system portion-wise. Sufficient precursor must be used to allow for materials transfer losses of diazomethane that are inevitable in the incomplete separation procedures described following. A yellow color will develop in the ether phase as the diazomethane is generated. After all of the precursor has been added, the solutions may be stirred for 10 min or so to allow the reaction to complete. The upper ether layer is decanted into a clean flask held in an ice-water bath. DO NOT use a separatory funnel with a ground-glass stopcock to separate the aqueous solution from the ether phase. Another portion of ether is added to the reaction flask, and it is stirred at ice-water bath temperature to extract remaining diazomethane. This ether layer is also decanted into the clean flask in the ice-water bath. This process may be repeated. The combined ether phases are likely to contain some dissolved water, which may be removed by adding KOH pellets and allowing the solution to stand in an ice-water bath for 0.5–3 h. The resulting yellow ethereal solution of diazomethane is ready for use. This procedure can be conducted on up to a 60 mmol scale. -
Definition
ChEBI: The simplest diazo compound, in which a diazo group is attached to a methylene group. -
Allgemeine Beschreibung
Yellow gas with a musty odor. Highly toxic by inhalation Shipped as a liquid under pressure. -
Air & Water Reaktionen
Reacts with water, releasing nitrogen, more stable in ether or dioxane. -
Reaktivität anzeigen
Diazomethane undergoes violent thermal decomposition. Above 200°C. the vapors may explode violently if rough glass surfaces are present. Explosions at low temperatures can occur if traces of organic matter are present. [J. Phys. Chem. 35:1403(1931)]. Produces explosions with alkali metals. Reacts with copper powder and to some extent all solid surfaces to produce nitrogen and solid white polymethylene. Reacts with dimethylaminodimethylarsine and trimethyltin in ether with vigorous foaming. -
Health Hazard
Diazomethane vapor causes severe irritation of the skin, eyes, mucous membranes, and lungs. It is considered to be a substance with poor warning properties, and the effects of exposure may be delayed in onset. Symptoms of exposure may include headache, chest pain, cough, fever, severe asthmatic attacks, and pulmonary edema, which can be fatal. Exposure of the skin and mucous membranes to diazomethane may cause serious burns. Diazomethane is a powerful allergen. Prolonged or repeated exposure to diazomethane can lead to sensitization of the skin and lungs, in which case asthma- like symptoms or fever may occur as the result of exposure to concentrations of diazomethane that previously caused no symptoms. Chronic exposure to diazomethane has been reported to cause cancer in experimental animals, but this substance has not been identified as a human carcinogen. Note that diazomethane is often prepared in situ from precursors that may themselves be highly toxic and/or carcinogenic. -
Health Hazard
It is a highly toxic gas and an irritant toeye, nose, and the entire respiratory tract.Exposure can cause dizziness, weakness,chest pain, severe headache, fever, asthmaticattack and pneumonia. Exposure to traceconcentrations of this substance can alsoproduce adverse effects, causing coughing,wheezing and headache. There have beenmany reported cases of poisoning. Its toxicitymay be attributed to its strong methylatingproperty. -
Brandgefahr
Pure diazomethane gas and liquid are readily flammable and can explode easily. A variety of conditions have been reported to cause explosions of diazomethane, including contact with rough surfaces such as ground-glass joints, etched or scratched flasks, and glass tubing that has not been carefully fire-polished. Direct sunlight and strong artificial light may also cause explosions of this substance. Violent reactions may occur on exposure of diazomethane to alkali metals. -
Flammability and Explosibility
Pure diazomethane gas and liquid are readily flammable and can explode easily. A variety of conditions have been reported to cause explosions of diazomethane, including contact with rough surfaces such as ground-glass joints, etched or scratched flasks, and glass tubing that has not been carefully fire-polished. Direct sunlight and strong artificial light may also cause explosions of this substance. Violent reactions may occur on exposure of diazomethane to alkali metals. -
Sicherheitsprofil
Confirmed carcinogen with experimental tumorigenic data. A poisonous irritant by inhalation. A powerful allergen. It can cause pulmonary edema and frequently causes hypersensitivity leading to asthmatic symptoms. Mutation data reported. Highly explosive when shocked, exposed to heat, or by chemical reaction. Undiluted liquid or gas may explode on contact with alkali metals, rough surfaces, heat (lOO°C), hgh-intensity light, or shock. When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of NOx. Incompatible with alkali metals; calcium sulfate. -
Synthese
To a solution of potassium hydroxide (30 mL,40%) in ether (100 ml), cooled below 5 °C with ice bath, was added in batches α-nitroso-α-methylurea with stirring. The organic phase was separated and dried over globosity potassium hydroxide for 3 hours. There is diazomethane (~2.6 g) in the ether solution, which was used without further purification. -
mögliche Exposition
Diazomethane is a powerful methylat- ing agent for acidic compounds, such as carboxylic acids, phenols and enols. It is used in pesticide manufacture and pharmaceutical manufacture. -
Erste Hilfe
.First Aid: If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Medical observation is recommendedfor 24-48 h after breathing overexposure, as pulmonaryedema may be delayed. As first aid for pulmonary edema,a doctor or authorized paramedic may consider administering a corticosteroid spray. If frostbite has occurred, seekmedical attention immediately; do NOT rub the affectedareas or flush them with water. In order to prevent furthertissue damage, do NOT attempt to remove frozen clothingfrom frostbitten areas. If frostbite has NOT occurred,immediately and thoroughly wash contaminated skin withsoap and water -
Carcinogenicity
Diazomethane was administered to rats and mice by inhalation, dermal, or subcutaneous injection routes using concentrations of 0.1 or 3.3 mg/mL. Mice developed lung tumors following either dermal application or inhalation at both concentrations. -
Lager
diazomethane should preferably be handled in solution using glassware specially designated for diazomethane (e.g., with Clear-Seal joints) and should be used as soon as possible after preparation. Storage of diazomethane solutions (even at low temperature) is not advisable. All work with diazomethane should be conducted in a fume hood behind a safety shield, and appropriate impermeable gloves, protective clothing, and safety goggles should be worn at all times. -
Versand/Shipping
UN1953 Compressed gas, toxic, flammable, n.o.s. -
Inkompatibilitäten
Heat (at about or above 100 C), shock, friction, concussion, sunlight, or other intense illuminations may cause explosions. Contact with alkali metals; drying agents such as calcium sulfate, or rough edges (such as ground glass) may cause explosions. Diazo compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. This chemical is sensitive to prolonged exposure to heat. This chemical is incompatible with strong oxidizing agents . -
Waste disposal
Decompose chemically with ceric ammonium nitrate under constant agitation and cooling . -
Vorsichtsmaßnahmen
Diazomethane is attractive as a methylating agent for carboxylic acids and phenols because it reacts quickly and highly efficiently with the production of only N2 as a by-product (Black, 1983). Its natural yellow color is discharged as it reacts, providing automatic indication of reaction progress. However, because diazomethane is highly toxic, it should be generated and used only in a well-functioning fume hood. Because it explodes on contact with some metals or ground glass of any type (joints, stoppers, syringes, stopcocks), it should be handled behind a safety shield, and other personal protective equipment should be used. Because it has a boiling point of ?23°C, it is usually handled in the ethereal solutions in which it is generated. Because it explodes on contact with CaSO4, its solutions or vapors must never be dried with drierite. Despite all of these hazards, it can be worked with safely, provided that appropriate precautions are observed.
Diazomethan Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(20)Suppliers
-
Henan Tianfu Chemical Co.,Ltd.
Telefon +86-0371-55170693<br/>+86-19937530512
E-Mail info@tianfuchem.com
-
Hubei xin bonus chemical co. LTD
Telefon 86-13657291602
E-Mail linda@hubeijusheng.com
-
Dideu Industries Group Limited
Telefon +86-29-89586680<br/>+86-15129568250
E-Mail 1026@dideu.com
-
Dayang Chem (Hangzhou) Co.,Ltd.
Telefon 571-88938639<br/>+8617705817739
E-Mail info@dycnchem.com
-
Telefon +86-85511178;<br/>+86-85511178;
E-Mail peter68@ptchemgroup.com
-
Telefon +86-0592-6210733
E-Mail sale@mainchem.com
-
Telefon +1-510-219-6317
E-Mail sales@hbcchem.com
-
Chizhou Kailong Import and Export Trade Co., Ltd.
Telefon
E-Mail xg01_gj@163.com
-
Jinan Yaoyan Pharmaceutical Co., Ltd.
Telefon
E-Mail jnyaoyan@163.com
-
Nanjing Meihao Pharmaceutical Technology Co., Ltd.
Telefon meitaochem@126.com
E-Mail meitaochem@126.com
1of2
