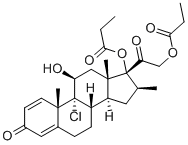
9-Chlor-11β-hydroxy-16β-methylpregna-1,4-dien-3,20-dion-17,21-dipropionat
Bezeichnung:9-Chlor-11β-hydroxy-16β-methylpregna-1,4-dien-3,20-dion-17,21-dipropionat
CAS-Nr5534-09-8
Englisch Name:Beclomethasone dipropionate
CBNumberCB4376831
SummenformelC28H37ClO7
Molgewicht521.04
MOL-Datei5534-09-8.mol
Synonyma
9-Chlor-11β-hydroxy-16β-methylpregna-1,4-dien-3,20-dion-17,21-dipropionat
9-Chlor-11β-hydroxy-16β-methylpregna-1,4-dien-3,20-dion-17,21-dipropionat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 117-120 C |
| alpha | D +98.0° (c = 1.0 in dioxane) |
| Siedepunkt | 613.3°C (rough estimate) |
| Dichte | 1.0766 (rough estimate) |
| Brechungsindex | 1.4429 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| Löslichkeit | Chloroform (Slightly), Dioxane (Slightly, Sonicated), Methanol (Slightly, Heated |
| Aggregatzustand | Solid |
| pka | 13.02±0.70(Predicted) |
| Farbe | White |
| maximale Wellenlänge (λmax) | 238nm(EtOH)(lit.) |
| Merck | 14,1019 |
| InChIKey | KUVIULQEHSCUHY-NHOFSDNKNA-N |
| SMILES | [C@@]1(OC(=O)CC)(C(=O)COC(=O)CC)[C@@H](C)C[C@@]2([H])[C@]3([H])CCC4=CC(=O)C=C[C@]4(C)[C@@]3(Cl)[C@@H](O)C[C@]12C |&1:0,14,17,19,29,31,33,36,r| |
| CAS Datenbank | 5534-09-8(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | T,Xn |
| R-Sätze: | 60-61-36/37/38-20/21/22 |
| S-Sätze: | 53-36/37/39-45-36-26 |
| WGK Germany | 3 |
| RTECS-Nr. | TU3805000 |
| HS Code | 29372100 |
| Toxizität | LD50 oral in rat: > 3750mg/kg |


