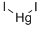
Quecksilberdiiodid
Bezeichnung:Quecksilberdiiodid
CAS-Nr7774-29-0
Englisch Name:MERCURY(II) IODIDE
CBNumberCB2469935
SummenformelHgI2
Molgewicht454.4
MOL-Datei7774-29-0.mol
Synonyma
Quecksilberdiiodid
Quecksilberdiiodid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 259 °C(lit.) |
| Siedepunkt | 354 °C(lit.) |
| Dichte | 6.36 |
| chüttdichte | 1350kg/m3 |
| Dampfdruck | 0.006 hPa (80 °C) |
| Flammpunkt | 350°C subl. |
| storage temp. | Store at RT. |
| Löslichkeit | potassium iodide solution: passes test |
| Aggregatzustand | beads |
| Wichte | 6.271 |
| Farbe | White |
| PH | 6-7 (50g/l, H2O, 20℃)(slurry) |
| Geruch (Odor) | Odorless |
| Wasserlöslichkeit | Insoluble inwater. Slightly soluble in alcohol, ether, acetone, chloroform, ethyl acetate, olive oil and castor oil. |
| Sensitive | Light Sensitive |
| Merck | 14,5879 |
| Solubility Product Constant (Ksp) | pKsp: 28.54 |
| Expositionsgrenzwerte | ACGIH: TWA 0.025 mg/m3; TWA 0.01 ppm (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Kennzeichnung gefährlicher | T+,N |
| R-Sätze: | 26/27/28-33-50/53 |
| S-Sätze: | 13-28-45-60-61 |
| RIDADR | UN 2025 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS-Nr. | OW5250000 |
| F | 8 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | II |
| HS Code | 28521000 |
| Giftige Stoffe Daten | 7774-29-0(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 18 mg/kg LD50 dermal Rat 75 mg/kg |



