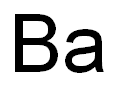
Barium
Bezeichnung:Barium
CAS-Nr7440-39-3
Englisch Name:Barium
CBNumberCB0854186
SummenformelBa
Molgewicht137.33
MOL-Datei7440-39-3.mol
Synonyma
Barium
Barium physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 725 °C(lit.) |
| Siedepunkt | 1640 °C(lit.) |
| Dichte | 3.6 g/mL at 25 °C(lit.) |
| storage temp. | water-free area |
| Löslichkeit | reacts with H2O; slightly soluble in ethanol |
| Aggregatzustand | rod |
| Wichte | 3.51 |
| Farbe | Silver-gray |
| PH | 0.5 (20°C in H2O) |
| Flame Color | Light apple green |
| Widerstand (resistivity) | 50.0 μΩ-cm, 20°C |
| Wasserlöslichkeit | soluble with H2 evolution in cold H2O and hot H2O; slightly soluble alcohol; insoluble benzene [CRC10] |
| Sensitive | air sensitive, moisture sensitive |
| Merck | 13,967 |
| Expositionsgrenzwerte | TLV-TWA 0.5 mg/m3 (for soluble compounds) (ACGIH and MSHA); IDLH (for soluble compounds) 250 mg/m3 (NIOSH). . |
| Stabilität | Stability Reacts vigorously or violently with acids, water, tetrachloromethane, small halogenated hydrocarbons. Should be stored under an inert material such as petroleum ether to exclude air. Flammable. |
| CAS Datenbank | 7440-39-3(CAS DataBase Reference) |
| EPA chemische Informationen | Barium (7440-39-3) |
| Kennzeichnung gefährlicher | C,Xi,F |
| R-Sätze: | 25-26-34-36/37/38-14/15-11 |
| S-Sätze: | 23-26-36-36/37/39-45-43-36/37-16 |
| RIDADR | UN 3264 8/PG 3 |
| OEB | C |
| OEL | TWA: 0.5 mg/m3 |
| WGK Germany | 3 |
| RTECS-Nr. | CQ8370000 |
| TSCA | Yes |
| HS Code | 2805 19 10 |
| HazardClass | 8 |
| PackingGroup | III |
| Giftige Stoffe Daten | 7440-39-3(Hazardous Substances Data) |
| Toxizität | An element; the heaviest of the stable alkaline earths. Barium sulfate is used as a diagnostic aid in radiology due to its radio-opaqueness and, because of its insolubility and lack of absorption, it is safe barring iatrogenic episodes. Poisoning usually results from deliberate or accidental ingestion of soluble barium compounds. The Ba2+ ion is a muscle poison due to the blocking of the K1 channels of the Na+/K+ pump in cell membranes. Because cases of barium poisoning are accompanied by severe hypokalemia, potassium infusion is an effective antidote. The toxicity of barium compounds depends on their solubility, with the free ion being readily absorbed from gastrointestinal tract or lung, whereas the sulfate is essentially unabsorbed. Thus, administration of soluble sulfates immediately after ingestion is another effective antidote. |
| IDLA | 50 mg Ba/m3 |



