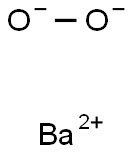
Bariumperoxid
Bezeichnung:Bariumperoxid
CAS-Nr1304-29-6
Englisch Name:Barium peroxide
CBNumberCB6853015
SummenformelBaO2
Molgewicht169.33
MOL-Datei1304-29-6.mol
Synonyma
Bariumperoxid
Bariumdioxid
Bariumsuperoxid
Bariumperoxid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 450 °C |
| Siedepunkt | losesO2 at 800°C |
| Dichte | 4,96 g/cm3 |
| Flammpunkt | 21 °C |
| Löslichkeit | reacts with dilute acid solutions |
| Aggregatzustand | Powder |
| Wichte | 4.96 |
| Farbe | White |
| PH | 12 (20°C, 10g/L in H2O, slurry) |
| Geruch (Odor) | Odorless |
| Wasserlöslichkeit | Insoluble in water |
| Sensitive | Moisture Sensitive |
| Merck | 14,989 |
| Stabilität | Stable. Strong oxidizer - contact with combustible material may cause fire. Incompatible with organic materials, combustible materials, reducing agents, most common metals. |
| LogP | -0.425 (est) |
| CAS Datenbank | 1304-29-6(CAS DataBase Reference) |
| EPA chemische Informationen | Barium peroxide (Ba(O2)) (1304-29-6) |
| Kennzeichnung gefährlicher | O,Xn |
| R-Sätze: | 8-20/22 |
| S-Sätze: | 13-27 |
| RIDADR | UN 1449 5.1/PG 2 |
| WGK Germany | 1 |
| RTECS-Nr. | CR0175000 |
| F | 3-9-23 |
| TSCA | Yes |
| HazardClass | 5.1 |
| PackingGroup | II |
| HS Code | 28164000 |
| Giftige Stoffe Daten | 1304-29-6(Hazardous Substances Data) |
| Toxizität | LD50 scu-mus: 50 mg/kg ZVKOA6 19,186,74 |



