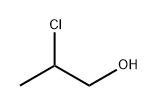
Chemical Properties
Colorless liquid; mild, nonresidual odor.
Soluble in water and alcohol.
Uses
In preparation of propylene oxide, q.v.
General Description
Clear colorless liquid with a pleasant odor.
Air & Water Reactions
Water soluble.
Reactivity Profile
2-Chloro-1-propanol may be sensitive to prolonged exposure to light. 2-Chloro-1-propanol can react with oxidizing agents. .
Fire Hazard
2-Chloro-1-propanol is flammable.
Hazard
Moderate fire risk. Toxic by ingestion and
skin absorption. Liver damage. Questionable car-
cinogen.
Potential Exposure
This material is used in organic
synthesis; to make other chemicals.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts
the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention
immediately. If this chemical has been inhaled, remove
from exposure, begin rescue breathing (using universal
precautions, including resuscitation mask) if breathing has
stopped and CPR if heart action has stopped. Transfer
promptly to a medical facility. When this chemical
has been swallowed, get medical attention. Give large
quantities of water and induce vomiting. Do not make
an unconscious person vomit.
Shipping
UN2611Propylene chlorohydrin, Hazard class:
6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.
Incompatibilities
Vapor may form explosive mixture
with air. Incompatible with oxidizers (chlorates, nitrates,
peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. Alcohols are sensitive to light and oxi-
dation. It is unstable in solution. It undergoes hydrolysis in
aqueous buffers. Contact with alkali metals, nitrides, and
strong reducing agents may form flammable and/or toxic
gases. May react with anhydrides forming acids and esters,
generating noticeable heat, and also with oxoacids and
carboxylic acids to form esters plus water, but the heat
of reaction in the latter case typically is low. May initiate
the polymerization of isocyanates and epoxides.
Waste Disposal
Dissolve or mix the
material with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber.
All federal, state, and local environmental regulations must
be observed.
Health Hazard
Propylene β-chlorohydrin is a moderatelytoxic compound having irritant actions onthe skin and eyes. Instilling 2.2 mg of liquidcaused severe eye irritation in rabbits. It cancause poisoning via ingestion, inhalation, andskin absorption. Exposure to 500 ppm for4 hours was lethal to rats. The lethal dosein dogs by oral intake was 200 mg/kg. Thetarget organs are the CNS, gastrointestinalsystem, liver, and kidney.
LD50 value, skin (rabbits): 529 mg/kg
Studies conducted to determine increasedrisks of pancreatic and lymphopoietic can cer among workers in ethylene and propylene chlorohydrin production remained inconclu sive and have given inconsistent findings(Olsen et al. 1997).