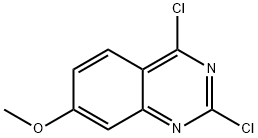
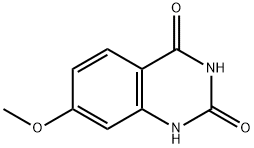
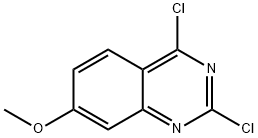
General procedure for the synthesis of 2,4-dichloro-7-methoxyquinazoline from 7-methoxy-2,4(1H,3H)-quinazoline dione: 7-methoxyquinazoline-2,4-diol (3 g, 15.6 mmol) was mixed with phosphorochloridic acid chloride (10 ml), and the reaction was stirred under reflux conditions overnight. After completion of the reaction, the mixture was cooled to room temperature, slowly poured into ice water and the pH was adjusted to 7-8 with sodium bicarbonate solution.Subsequently, the aqueous layer was extracted with dichloromethane, the organic layers were combined and dried with anhydrous magnesium sulfate. After the solvent was removed by concentration under reduced pressure, the residue was purified by silica gel column chromatography (eluent ratio: n-hexane/ethyl acetate=10/1) to afford the target product 2,4-dichloro-7-methoxyquinazoline (0.64 g) as a white solid.1H NMR (400 MHz, CDCl3) δ 8.12 (d, 1H), 7.37-7.20 (m, 2H) 3.99 (s, 3H).