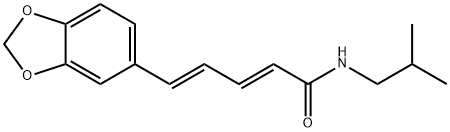
Description
Piperlonguminine is an alkaloid amide from species of the genus
Piper, a plant used in traditional medicine that demonstrates antifungal, anticancer, antihyperlipidemic, and anti-
inflammatory properties.
1,2 Piperlonguminine (3-
12.5 μM) has been shown to dose dependently decrease expression of amyloid precursor protein and amyloid-β peptide in human neuroblastoma cells, suggesting it may have implication in treating Alzheimer’s disease.
3
Description
This amide alkaloid also occurs in the roots of Piper longum and yields colourless
needles from EtOH. The ultraviolet spectrum exhibits absorption maxima at 245,256, 307 and 340 mil. The side chain is doubly unsaturated and on catalytic
hydrogenation, the base furnishes the tetrahydro derivative, m.p. 66°C.
Definition
ChEBI: (E,E)-Piperlonguminine is a member of benzodioxoles.
Biological Activity
Piperlonguminine is an alkaloid amide isolated from the Piper species. It has a wide range of biological properties including anti-inflammatory, anti-tumour, neuroprotective, anti-platelet, anti-melanogenic, anti-fungal and antibacterial activities. In addition, its derivative piperlonguminine acid has Antihyperlipidemic activity.
in vitro
in a previous study, piperlonguminine was discovered to inhibit melanin production in melanoma b16 cells stimulated with α-msh, 3-isobutyl-1-methylxanthine or protoporphyrin ix, where piperlonguminine showed stronger depigmenting efficacy. however, piperlonguminine could not alter1-oleoyl-2-acetyl-sn-glycerol-induced melanogenesis and could not affect protein kinase c-mediated melanin production. in additioin, piperlonguminine was not able to inhibit the catalytic activity of cell-free tyrosinase from melanoma b16 cells, and such effect was attributed to the inhibitory action of piperlonguminine on α-msh-induced signaling via camp to the camp responsive element binding protein [1].
in vivo
in vivo, rats were subjected to middle cerebral artery occlusion for 1h, followed by reperfusion for 23 h. the results showed that the intraperitoneal injection of piperlonguminine pe at 2.4 mg/kg was able to produce a significant neuroprotective potential in rats with cerebral ischemia. in addition, piperlonguminine could attenuate the neurological deficit scores, brain infarct volume and brain water content, and could inhibit the activation of nf-κb and mapk [2].
References
Chatterjee, Dutta., Tetrahedron Lett., 1797 (1966)
Chatterjee, Dutta., Tetrahedron, 23, 1769 (1967)