Synthesis
1. Acetyl chloride (21.32 mL, 300 mmol) was slowly added dropwise to 200 mL of anhydrous methanol under ice bath conditions with stirring for 30 minutes.
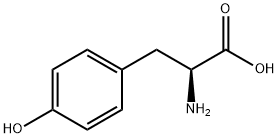
2. L-tyrosine (18.1 g, 100 mmol) was added to the above solution, followed by transferring the reaction mixture to an oil bath and heating to reflux for 4 hours.
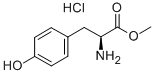
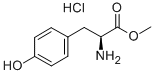
3. After completion of the reaction, the solvent was removed by distillation under reduced pressure. Appropriate amount of acetone was added to the residue and filtered to obtain white powdery L-tyrosine methyl ester hydrochloride (22.78 g, 99% yield).
4. Di-tert-butyl dicarbonate (11.34 g, 52 mmol) was dissolved in 50 mL of 1,4-dioxane and slowly added to 160 mL of mixed solvent (1,4-dioxane-H2O = 1:1) containing L-tyrosine methyl ester hydrochloride (9.26 g, 40 mmol) and K2CO3 (8.28 g, 60 mmol).
5. After stirring the reaction mixture at room temperature overnight, the solvent was removed by concentration under reduced pressure. EtOAc was added for extraction and the EtOAc layer was washed sequentially with 1N citric acid, saturated NaHCO3 solution and brine.
6. The organic layer was dried over anhydrous MgSO4 and concentrated under reduced pressure to give compound 2 in white powder form, which can be used in subsequent steps without further purification.
References
[1] Synthetic Communications, 1992, vol. 22, # 7, p. 979 - 985
[2] Tetrahedron, 2001, vol. 57, # 39, p. 8313 - 8322
[3] Chemistry - A European Journal, 2011, vol. 17, # 22, p. 6206 - 6213
[4] European Journal of Medicinal Chemistry, 2018, vol. 145, p. 649 - 660
[5] Heterocycles, 2002, vol. 56, # 1-2, p. 157 - 170