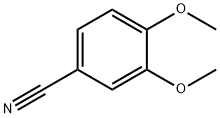
2024-83-1
| Name | 2,3-DIMETHOXYBENZONITRILE |
| CAS | 2024-83-1 |
| EINECS(EC#) | 217-969-2 |
| Molecular Formula | C9H9NO2 |
| MDL Number | MFCD00001784 |
| Molecular Weight | 163.17 |
| MOL File | 2024-83-1.mol |
Chemical Properties
| Appearance | white to light yellow crystal powder |
| Melting point | 68-70 °C(lit.) |
| Boiling point | 290.25°C (rough estimate) |
| density | 1.2021 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| Fp | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in methanol. |
| form | powder to crystal |
| color | White to Light yellow |
| BRN | 1451651 |
| InChI | 1S/C9H9NO2/c1-11-8-4-3-7(6-10)5-9(8)12-2/h3-5H,1-2H3 |
| InChIKey | OSEQIDSFSBWXRE-UHFFFAOYSA-N |
| SMILES | COc1ccc(cc1OC)C#N |
| CAS DataBase Reference | 2024-83-1(CAS DataBase Reference) |
Safety Data
| Hazard Codes | Xn,T,Xi |
| Risk Statements | |
| Safety Statements | |
| RIDADR | 3276 |
| WGK Germany | 3 |
| RTECS | DI4355000 |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 2926907090 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Dermal Acute Tox. 4 Inhalation Acute Tox. 4 Oral Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |