What is Pyrrolidine?
Feb 20,2020
Pyrrolidine appears as a colorless to pale yellow liquid with an ammonia-like odor. Vapors heavier than air. Produces toxic oxides of nitrogen during combustion.
Pyrrolidine is a cyclic amine whose five-membered ring contains four carbon atoms and one nitrogen atom; the parent compound of the pyrrolidine family. It is a saturated organic heteromonocyclic parent, a member of pyrrolidines and an azacycloalkane. It is a conjugate base of a pyrrolidinium ion[1].
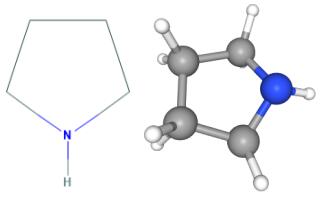
Fig 1. Chemical structure formula and three-dimensional structure of pyrrolidine
Pyrrolidine, one of biogenic volatile amines, possesses nicotine-like synaptotropic actions on the nervous systems. Pyrrolidine levels in the tissue were examined by using mass fragmentographic technique. High concn of pyrrolidine were found in the seminal vesicle and lung of rabbits. Only trace amt of pyrrolidine existed in the brain of mice and rats, although higher concn were detected in the brain of rabbits. In the rat brain, however, high levels of pyrrolidine were found in the pineal gland, pituitary gland and corpus striatum[2].
Pyrrolidine can be produced from butanediol and ammonia, e.g., over an aluminum thorium oxide catalyst at 300℃ or over a nickel catalyst at 200℃ and 20 MPa under hydrogenation conditions. It can also be produced from tetrahydrofuran and ammonia over aluminum oxide at 275-375℃[3].Production can be obtained by reaction of 1,4-dihydroxyalkanes with amines in the presence of dehydrating agents at elevated temperatures or by reaction of primary amines with 1,4-dihaloalkanes. The dry distillation of 1,4-butanediamine dihydrochloride also generates pyrrolidine[4].
Pyrrolidine's production and use in the synthesis of drugs and antibiotics and in vulcanization accelerators may result in its release to the environment through various waste streams. Naturally occurring sources of pyrrolidine can be found in vegetables, dairy products, cigarettes, alcoholic beverages and coffee. If released to air, a vapor pressure of 62.7 mm Hg at 25℃ indicates pyrrolidine will exist solely as a vapor in the atmosphere. Vapor-phase pyrrolidine will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 5 hours. Pyrrolidine does not contain chromophores that absorb at wavelengths >290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight. If released to soil, pyrrolidine is expected to have very high mobility based upon an estimated Koc of 42. However, the pKa of pyrrolidine is 11.31, indicating that this compound will primarily exist in the cation form in the environment and cations generally adsorb more strongly to soils containing organic carbon and clay than their neutral counterparts. Volatilization from moist soil surfaces is not expected to be an important fate process as cations do not volatilize. Pyrrolidine may volatilize from dry soil surfaces based upon its vapor pressure. If released into water, pyrrolidine is not expected to adsorb to suspended solids and sediment based upon the estimated Koc. Pyrrolidine was found to degrade anaerobically via denitrification in 7-15 days in microbial consortia from freshwater sediments, estuarine sediments and activated sludge. A pKa of 11.31 indicates pyrrolidine will exist almost entirely in the cation form at pH values of 5 to 9 and therefore volatilization from water surfaces is not expected to be an important fate process. An estimated BCF of 3 suggests the potential for bioconcentration in aquatic organisms is low. Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions. Occupational exposure to pyrrolidine may occur through inhalation and dermal contact with this compound at workplaces where pyrrolidine is produced or used. Monitoring and use data indicate that the general population may be exposed to pyrrolidine via ingestion of food and drinking water, use of tobacco products, and dermal contact with this compound and other products containing pyrrolidine.
References
[1]Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 14th Edition. John Wiley & Sons, Inc. New York, NY 2001., p. 943.
[2]Okano Y , Miyata T , Iwasaki K , et al. Analysis of endogenous pyrrolidine levels by mass fragmentography[J]. Life Sciences, 1982, 31(7):671-677.
[3]Collin G, Höke H. Naphthalene and Hydronaphthalenes[M]// Ullmann's Encyclopedia of Industrial Chemistry. 2000.
[4]Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V20: 702 (1996)
You may like
The potential risk of 1,2-hexanediol
Mar 11, 2025
Is Methyl eugenol an insecticide?
Dec 16, 2024
Related articles And Qustion
Lastest Price from Pyrrolidine manufacturers
Pyrrolidine

US $10.00/ASSAYS2025-04-21
- CAS:
- 123-75-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 tons
Pyrrolidine
US $100.00/Kg/Drum2025-04-21
- CAS:
- 123-75-1
- Min. Order:
- 1Kg/Drum
- Purity:
- 99%min
- Supply Ability:
- 200TON