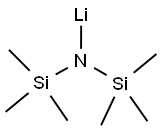
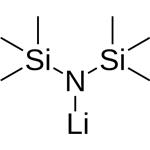
What is Lithium bis(trimethylsilyl)amide?
Identification
Product Name: Lithium bis(trimethylsilyl)amide
Synonyms: 1,1,1,3,3,3-HEXAMETHYLDISILAZAN LITHIUMSALZ;1,1,1,3,3,3-HEXAMETHYLDISILAZANE LITHIUM SALT;HEXAMETHYLDISILAZANE LITHIUM SALT;LHMDS;LIHMDS;LITHIUM HEXAMETHYLDISILAZANE;LITHIUM HEXAMETHYLDISILAZIDE;LITHIUM-BIS-(TRIMETHYLSILYL)-AMID
CAS: 4039-32-1
MF: C6H18LiNSi2
MW: 167.33
EINECS: 223-725-6
Properties
Melting point −55 °C(lit.)
Boiling point 120-122 °C(lit.)
Density 0.775 g/mL at 20 °C(lit.)
Vapor density 4 (vs air)
Vapor pressure 21 hPa (20 °C)
Refractive index n20/D 1.4179(lit.)
Fp 50 °F
Storage temp. Store at RT.
Solubility H2O: 10 mg/mL at 20 °C, clear, colorless
Form Liquid
Pka 10.40, 8.26(at 25℃)
Color Clear colorless to slightly yellow
General Description
Lithium bistrimethylsilylamide is also called lithium hexamethylsilylamide (LiHMDS). Lithium bistrimethylsilylamide can be used to prepare low-coordination metal complexes because the ligand (TMS) 2N- has a large steric hindrance. Such examples are M[N(TMS)2]3 (M=Sc,Ti,V,Fe; TMS=(CH3)3SChemicalbooki)). It reacts with trimethylchlorosilane to produce tris(trimethylsilyl)amine, in which the coordination number of nitrogen is 3, and the spatial configuration is a plane regular triangle. In organic synthesis or pharmaceutical synthesis, lithium bistrimethylsilylamide is usually used as a strong base to form various lithium salts through exchange reactions, such as lithium acetylene, or lithium enol salts, etc. to further complete the conversion of functional groups.
Application
Lithium bistrimethylsilylamide (LiHMDS) can be used as a strong non-nucleophilic base and can be used in the reaction to form enols. The stereospecific Claisen rearrangement reaction of enol esters converts aldehydes into silimides. The reaction, the reaction of glycidyl ester, the reaction of β-lactam, the selective cleavage reaction of aryl alkyl ether, are mainly used in laboratory research and development process and organic synthesis process.
Lithium bis(trimethylsilyl)amide is a non-nucleophilic strong Brønsted base, which is generally soluble in most of the nonpolar organic solvents. It is most commonly employed in organic reactions. It is used as the base employed in generating enolates for the preparation of lactone precursors, pyranones, and cyclohexanes. Used to catalyze the addition of phosphine P-H bonds to carbodiimides leading to phosphaguanidines. Also used in a novel three-step synthesis of disubstituted 1,2,5-thiadiazoles.
Preparation
Take a clean and dry 500L reactor , the pressure gauge and thermometer are within the validity period, the bottom valve has been closed, and the reactor is replaced with argon three times; 180kg of tetrahydrofuran and 100kg of hexamethyldisilazane are added to the reactor through the upper tank, Cool down to -15℃±5℃, keep this temperature, open the balance pipe valve on Chemicalbook, and add 171kg of n-butyllithium solution (2.5M n-hexane solution) with a mass of 171kg and a molar concentration of 1.6M-2.5M through the upper tank. After dripping in about 6h, the temperature was raised to 10℃ and kept for 1h to obtain the lithium hexamethyldisilazide solution. The concentration was checked by sampling, and the yield was 98%.
See also
Lastest Price from Lithium bis(trimethylsilyl)amide manufacturers
US $0.00/kg2025-09-19
- CAS:
- 4039-32-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $10.00/ASSAYS2025-08-21
- CAS:
- 4039-32-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton