What is Aluminium hydroxide used for?
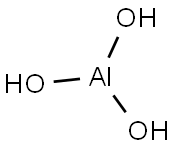
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic and acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina (Al2O3), the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite.

Production
Red mud reservoirs (this one in Stade, Germany) contain the corrosive residues from the production of aluminum hydroxide.
Virtually all the aluminium hydroxide used commercially is manufactured by the Bayer process which involves dissolving bauxite in sodium hydroxide at temperatures up to 270 °C (518 °F). The waste solid, bauxite tailings, is removed and aluminium hydroxide is precipitated from the remaining solution of sodium aluminate. This aluminium hydroxide can be converted to aluminium oxide or alumina by calcination.
The residue or bauxite tailings, which is mostly iron oxide, is highly caustic due to residual sodium hydroxide. It was historically stored in lagoons; this led to the Ajka alumina plant accident in 2010 in Hungary, where a dam bursting led to the drowning of nine people. An additional 122 sought treatment for chemical burns. The mud contaminated 40 square kilometres (15 sq mi) of land and reached the Danube. While the mud was considered non-toxic due to low levels of heavy metals, the associated slurry had a pH of 13.
Uses
Fire retardant filler
Aluminium hydroxide also finds use as a fire retardant filler for polymer applications. It is selected for these applications because it is colorless (like most polymers), inexpensive, and has good fire retardant properties.Magnesium hydroxide and mixtures of huntite and hydromagnesite are used similarly It decomposes at about 180 °C (356 °F), absorbing a considerable amount of heat in the process and giving off water vapour. In addition to behaving as a fire retardant, it is very effective as a smoke suppressant in a wide range of polymers, most especially in polyesters, acrylics, ethylene vinyl acetate, epoxies, PVC and rubber.
Precursor to Al compounds
Aluminium hydroxide is a feedstock for the manufacture of other aluminium compounds: speciality calcined aluminas, aluminium sulfate, polyaluminium chloride, aluminium chloride, zeolites, sodium aluminate, activated alumina, and aluminium nitrate.
Freshly precipitated aluminium hydroxide forms gels, which are the basis for the application of aluminium salts as flocculants in water purification. This gel crystallizes with time. Aluminium hydroxide gels can be dehydrated (e.g. using water-miscible non-aqueous solvents like ethanol) to form an amorphous aluminium hydroxide powder, which is readily soluble in acids. Heating converts it to activated aluminas, which are used as desiccants, adsorbent in gas purification, and catalyst supports.
Pharmaceutical
Under the generic name "algeldrate", aluminium hydroxide is used as an antacid in humans and animals (mainly cats and dogs). It is preferred over other alternatives such as sodium bicarbonate because Al(OH)3, being insoluble, does not increase the pH of stomach above 7 and hence, does not trigger secretion of excess acid by the stomach. Brand names include Alu-Cap, Aludrox, Gaviscon or Pepsamar. It reacts with excess acid in the stomach, reducing the acidity of the stomach content, which may relieve the symptoms of ulcers, heartburn or dyspepsia. Such products can cause constipation, because the aluminium ions inhibit the contractions of smooth muscle cells in the gastrointestinal tract, slowing peristalsis and lengthening the time needed for stool to pass through the colon.[21] Some such products are formulated to minimize such effects through the inclusion of equal concentrations of magnesium hydroxide or magnesium carbonate, which have counterbalancing laxative effects.
This compound is also used to control hyperphosphatemia (elevated phosphate, or phosphorus, levels in the blood) in people and animals suffering from kidney failure. Normally, the kidneys filter excess phosphate out from the blood, but kidney failure can cause phosphate to accumulate. The aluminium salt, when ingested, binds to phosphate in the intestines and reduce the amount of phosphorus that can be absorbed.
Side effects
Common side effects or health problems may include:
Nausea
Vomiting
Rebound hyperacidity
Aluminum-intoxication
Low blood phosphates (hypophosphatemia)
Chalky taste
Constipation (this could lead to hemorrhoids or bowel obstruction)
Fecal impaction
Stomach cramps
Milk-alkali syndrome
Softening of the bones
Serious side effects of aluminum hydroxide include::
Black/tarry stools
Mental/mood changes (e.g., confusion, deep sleep)
Pain with urination
Stomach/abdominal pain
Vomit that looks like coffee grounds
);You may like
Related articles And Qustion
Lastest Price from Aluminum hydroxide manufacturers

US $10.00/kg2024-04-24
- CAS:
- 21645-51-2
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 10000ton

US $50.00-1.00/KG2024-03-25
- CAS:
- 21645-51-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available