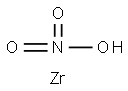What is Zirconium nitrate?
Zirconium nitrate is an inorganic compound with the chemical formula Zr(NO3)4. There are pentahydrate and anhydrate. Like other nitrates, zirconium nitrate is an oxidizing agent. Zirconium nitrate is white crystals and easily absorbs moisture. Decompose when heated to 100°C to produce zirconyl nitrate and nitric acid. Easily soluble in water, the aqueous solution is acidic and soluble in alcohol. The newly prepared zirconium dioxide hydrate is dissolved in the nitric acid and acts on it to obtain the coordination compound zirconia nitrate. Zirconium nitrate can be used as a reagent and preservative for the determination of fluoride, as well as for the separation of phosphate. In addition, zirconium nitrate is also an important catalyst in organic chemical industry.

Preparation
It is made by the action of zirconium hydroxide and nitric acid. Take 100 kilograms of zirconium oxychloride and add water to dissolve it to 24°C. Take another 35 kg of soda ash, add water to dissolve it to 24 ℃. Gradually add, and keep stirring to make PH=2.6. If it is not accurate, adjust it with zirconium oxychloride solution or soda lye respectively, and then clean it until it does not contain sodium chloride as the end point (indicator uses silver nitrate). After the obtained white zirconium carbonate is pressed and dried, for every 100 kg of zirconium carbonate by weight, add 10 kg of 98% nitric acid and slowly dissolve. The pH of the solution is 0.7~1.0, which contains 16% of zirconium dioxide, other iron, Titanium, silicon and heavy metals are 1%.
You may like
See also
Lastest Price from Zirconium nitrate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 13746-89-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 13746-89-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons