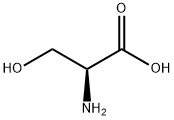
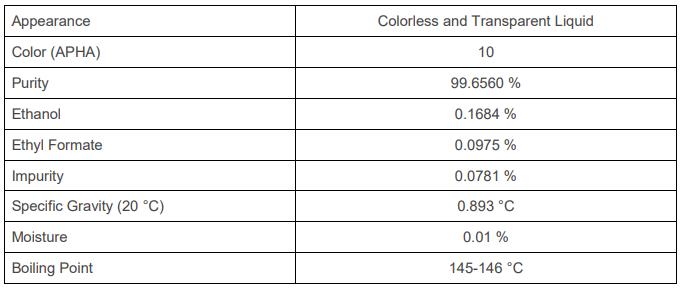
Structure and function of L-serine
L-serine is the L-enantiomer of serine. It has a role as a human metabolite, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a serine family amino acid, a proteinogenic amino acid, a L-alpha-amino acid and a serine. It is a conjugate base of a L-serinium. It is a conjugate acid of a L-serinate. It is an enantiomer of a D-serine. It is a tautomer of a L-serine zwitterion.

Structure and function
L-serine is an alpha-amino acid. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). Amino acids are organic compounds that contain amino (-NH2) and carboxyl (-COOH) functional groups, along with a side chain (R group) specific to each amino acid. L-serine is one of 20 proteinogenic amino acids, i.e., the amino acids used in the biosynthesis of proteins. Serine is found in all organisms ranging from bacteria to plants to animals. It is classified as a polar, uncharged (at physiological pH), aliphatic amino acid. In humans, serine is a nonessential amino acid that can be easily derived from glycine. A non-essential amino acid is an amino acid that can be synthesized from central metabolic pathway intermediates in humans and is not required in the diet. Like all the amino acid building blocks of protein and peptides, serine can become essential under certain conditions, and is thus important in maintaining health and preventing disease.
Source
L-Serine may be derived from four possible sources: dietary intake; biosynthesis from the glycolytic intermediate 3-phosphoglycerate; from glycine; and by protein and phospholipid degradation. Little data is available on the relative contributions of each of these four sources of l-serine to serine homoeostasis. It is very likely that the predominant source of l-serine will be very different in different tissues and during different stages of human development.
L-serine is the predominant source of one-carbon groups for the de novo synthesis of purine nucleotides and deoxythymidine monophosphate. It has long been recognized that, in cell cultures, L-serine is a conditional essential amino acid, because it cannot be synthesized in sufficient quantities to meet the cellular demands for its utilization. In recent years, L-serine and the products of its metabolism have been recognized not only to be essential for cell proliferation, but also to be necessary for specific functions in the central nervous system.
Mechanism
In the biosynthetic pathway, the glycolytic intermediate 3-phosphoglycerate is converted into phosphohydroxypyruvate, in a reaction catalyzed by 3-phosphoglycerate dehydrogenase (3- PGDH; EC 1.1.1.95).
Phosphohydroxypyruvate is metabolized to phosphoserine by phosphohydroxypyruvate aminotransferase (EC 2.6.1.52) and, finally, phosphoserine is converted into l-serine by phosphoserine phosphatase (PSP; EC 3.1.3.3). In liver tissue, the serine biosynthetic pathway is regulated in response to dietary and hormonal changes. Of the three synthetic enzymes, the properties of 3-PGDH and PSP are the best documented. Hormonal factors such as glucagon and corticosteroids also influence 3-PGDH and PSP activities in interactions dependent upon the diet.
You may like
Related articles And Qustion
Lastest Price from L-Serine manufacturers

US $0.00-0.00/kg2025-05-12
- CAS:
- 56-45-1
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 10 tons

US $0.00/Kg/Drum2025-04-21
- CAS:
- 56-45-1
- Min. Order:
- 1KG
- Purity:
- 98.5%-101.5%
- Supply Ability:
- 10 TONS