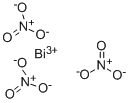
Properties and Uses of Bismuth nitrate
Bismuth nitrate is an inorganic compound, which is a colorless or white nitrate-like solid. It is easily deliquescent, easily soluble in nitric acid, glycerin, ethylene glycol, acetone, dilute acid, insoluble in ethanol, carbon tetrachloride and ethyl acetate. It decomposes into bismuth subnitrate in presence of water. Its molecular formula is Bi(NO3)3•5H2O, and bismuth nitrate with no crystal water has not been obtained.

Bismuth Nitrate Pentahydrateis a crystalline Bismuth source for uses compatible with nitrates and lower (acidic) pH. All metallic nitrates are inorganic salts of a given metal cation and the nitrate anion. The nitrate anion is a univalent (-1 charge) polyatomic ion composed of a single nitrogen atom ionically bound to three oxygen atoms (Formula: NO3) for a total formula weight of 62.05. Nitrate compounds are generally soluble in water.Bismuth nitrate is corrosive to the skin and direct contact with the skin should be avoided.
Nitrate materials are oxidizing agents. When mixed with hydrocarbons, nitrate compounds can form a flammable mixture. Nitrates are excellent precursors for production of ultra high purity compounds and certain catalyst and nanoscale (nanoparticles and nanopowders) materials. Bismuth Nitrate is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. We also produce Bismuth Nitrate Solution.
Bismuth nitrate is a catalyst which can be used to prepare aromatic amines by catalytic reduction of aromatic nitro compounds with activated carbon with the yield of between 78 and 99%. It is also used in the manufacture of other bismuth salts which are used for picture tubes and glazing paints. Basic salts are used as drugs. Bismuth subnitrate is soluble in dilute hydrochloric acid and nitric acid and insoluble in water and ethanol. Slightly hygroscopic.
When heated to red heat, it decomposes into antimony trioxide and nitrogen oxides. After oral administration, a protective film is formed on the gastric mucosa wound to alleviate the stimulation of food on the gastric mucosa, and sputum binds with hydrogen sulfide in the intestine to form insoluble bismuth sulfide (Bi2S3) on the intestinal mucosa to slow the intestinal peristalsis. It also has convergence, protection of gastric mucosa and antibacterial effects. It can be obtained by partial hydroxylation of cerium nitrate, then being filtered, concentrated, cooled and crystallized, solid-liquid separation and dried.
References
Clay, V.E., Fahmy, M.A.H., Martin, J.P. and Fukuto, T.R. (1980) J. Agric. Food Marsden, O.J., Kuwano, E. and Fukuto, T.R. (1982) Pestic. Biochem. Physiol., 18, 3848.
Umetsu, N., Fahmy, M.A.H. and Fukuto, T.R. (1979) Pestic. Biochem. Physiol., 10,
Umetsu, N., Kuwano, E. and Fukuto, T.R. (1980) J. Environ. Sci. Health, B15,l-23.