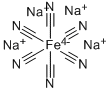
Preparation method of sodium ferrocyanide
It has been proposed to make sodium ferricyanide by electrolytic oxidation of sodium ferrocyanide. Such an operation may be carried out in a cell equipped with an anode and a cathode surrounded by a porous ceramic diaphragm forming the cathode chamber. On passage of current thru the cell, production of sodium ferricyanide is understood to take place as follows: (1) 2Na4Fe(CN)6+2HOH2Na3Fe(CN) 6+2NaOH+H2 According to this equation, it will be noted that as oxidation proceeds, for every mol of sodium ferricyanide formed one mol of NaOH is also produced. Approximately half of this NaOH is retained in the cathode chamber while the balance of such NaOH works thru the porous walls of the diaphragm into the anolyte solution which, on completion of electrolysis, is withdrawn from the cell.
In an operation of this nature, it is not feasible to carry electrolysis to a point where substantially all of the sodium ferrocyanide is oxidized to ferricyanide. Hence, a so-called finished sodium ferricyanide anolyte solution contains as an impurity, in addition to a substantial amount of sodium hydroxide, a generally equal quantity of unoxidized sodium ferrocyanide. To my knowledge substantially pure sodium ferricyanide, either in liquid or solid form, has not been commercially obtainable. Basis for this difficulty is the very high solubility of sodium ferricyanide coupled with the relatively high solubility of the sodium hydroxide and sodium ferrocyanide impurities.

The principal object of this invention is provision of a process for making sodium ferricyanide of controllable degree of purity. The invention aims to provide for electrolytic manufacture of sodium ferricyanide by procedure according to which it is possible to remove, substantially completely or to any desired lesser extent, the sodium hydroxide impurity without introducing extraneous impurities into the sodium ferricyanide liquor and without requiring losses of sodium or cyanide values. A further object lies in the provision of a process by practice of which it is possible to similarly remove, substantially completely or to any desired smaller degree, the sodium ferrocyanide impurity inherently present in a sodium ferricyanide anolyte formed by commercially feasible electrolytic oxidation of sodium ferrocyanide.
I have found that the objects of the invention, with respect to removal of sodium hydroxide impurity contained in a sodium ferricyanide solution, may be accomplished by treating the anolyte liquor containing sodium hydroxide with certain agents which function to convert the sodium of the sodium hydroxide to sodium iron-cyanide compounds which are soluble and remain in solution in the treated liquor, and to convert the hydroxide component of the sodium hydroxide to an iron hydroxide precipitate which may be removed from the treated solution by simple filtration. Thus, I am enabled to substantially completely neutralize or lower the sodium hydroxide content of anolyte liquors to any desired extent, to suit purity requirements of the sodium ferricyanide product, and to transform the sodium constituent of the sodium hydroxide to a soluble sodium iron-cyanide and remove from the system the hydroxide radical of the sodium hydroxide so neutralized, all without introducing into the operation any material which adversely affects purity of the ultimate product, and without causing loss of sodium or cyanide values by liquor bleed-off as is often necessary in chemical processes.
With regard to removal of sodium ferrocyanide impurity still present in the sodium ferricyanide liquor after the desired neutralization of sodium hydroxide, I find that there are certain hereinafter described conditions according to which the sodium ferricyanide solution may be concentrated in such a way that it becomes possible to precipitate out of the solution, in a readily separable form, substantially all or any desired lesser amount of the sodium ferrocyanide impurity, and to accomplish this separation without any substantial formation of solid ferricyanide, i. e., substantially all of the latter being held in solution.
.!0 Briefly, the process of the invention comprises electrolytically oxidizing a solution ofsodium ferrocyanide to form a sodium ferricyanide liquor containing sodium hydroxide and sodium ferro5 cyanide as impurities, treating the liquor with certain neutralizing agents to convert the hydroxide component of the sodium hydroxide to iron hydroxide, removing the latter from the system, concentrating resulting sodium ferricyanide liquor still containing soluble ferrocyanide impurity according to certain procedural steps to effect precipitation of sodium ferrocyanide impurity as crystals, separating the latter from the sodium ferricyanide liquor, and.then recovering, from the residual sodium ferricyanide solution, substantially pure sodium ferricyanide optionally in liquid or solid form.
In carrying out the invention, in apparatus such as illustrated diagrammatically on the accompanying flow sheet, when starting operation a sodium ferrocyanide solution may be formed in make-up tank 10, by dissolving in water Na4Fe(CN)6.10H20 crystals in quantity to form a substantially saturated solution. A sodium ferrocyanide solution as fed into the anolyte chamber II of an electrolytic cell 12 should contain a certain amount of sodium hydroxide, the function of which is primarily to prevent corrosion of the anode and secondarily to promote conductivity of the anolyte solution. Hence, a desired amount of sodium hydroxide from an external source may be added to the sodium ferrocyanide solution in tank 10, and a typical starting solution may comprise from 200 g. p. 1. to 300 g. p. 1. of Na4Fe(CN)6 and from one to 5 g. p. 1. of NaOH.
The starting solution is run into the anolyte chamber of the cell provided preferably with a nickel anode and a steel cathode surrounded by a porous ceramic diaphragm 13 forming a cathode chamber 14. Circulation of solution in the anolyte chamber, and solution temperature of the order of 40-500 C. may be maintained by any convenient means. Ordinarily, during the progress of oxidation, temperature should not exceed 50° C. in order to prevent decomposition of ferricyanide to iron hydroxide, and the temperature may be kept as high as say 400 C. to avoid crystallization of sodium ferricyanide.
The cathode chamber is filled with a weak solution of NaOH which functions primarily as a conductor between the cathode and the walls of the surrounding diaphragm. When the current is turned on, reaction proceeds in accordance with Equation (1), and sodium ferricyanide is formed. As previously explained, approximately half of the NaOH formed during the course of reaction works its way thru the porous wall of the diaphragm and into the anolyte solution.
The electrolytic oxidation operation may be carried out so as to form an anolyte solution relatively concentrated with respect to sodium ferricyanide. This may be accomplished by addition to the anolyte liquor, from time to time as oxidation proceeds, of further sodium ferrocyanide crystals in quantity to keep the anolyte solution substantially saturated with respect to sodium ferrocyanide up to a convenient point prior to completion of cell operation. A typical finished anolyte solution may contain from 300 to 350 g. p. 1. of Na3Fe(CN) 6, from 20 to 30 g. p. 1. of Na4Fe(CN)6, and from 20 to 30 g. p. 1. of NaOH. According to one example of practice of the process, the anolyte solution at the end of electrolysis may analyze 332 g. p. 1. of Na3Fe(CN)s, 24 g. p. 1. of Na4Fe(CN)6, and 23 g. p. 1. of NaOH. A feature of the present process is directed toward partial or substantially complete removal from the system of the sodium hydroxide which is contained in the finished anolyte solution and which was produced during oxidation. To this end, on completion of electrolysis, the anolyte solution is run into tank 16 for treatment.
Practice of this purification step comprises treating a liquor, of the type described and containing sodium hydroxide with a treating agent reactable with sodium hydroxide to form soluble sodium iron-cyanide and to precipitate iron hydroxide. More particularly I find that suitable treating agents are iron salts, of an acid of the group consisting of ferro and ferri cyanic acids, such salts being reactable with the sodium hydroxide contained in the liquors treated to form soluble sodium iron-cyanide and to pre. cipitate iron hydroxide. Examples of suitable neutralizing or treating agents are Prussian blue, understood to be ferric ferrocyanide, Fe4(Fe(CN) )s3; Turnbull's blue, understood to be ferrous ferricyanide, Fe3(Fe(CN)6)2; and lu a more or less white iron salt, understood to be ferrous ferrocyanide, Fe2Fe(CN)6.
The most satisfactory treating or neutralizing agent is Prussian blue which when added to a sodium ferricyanide solution containing sodium hydroxide reacts with sodium hydroxide in accordance with the following: (2) 12NaOH+Fe4(Fe(CN) 6)34Fe(OH) 3+3Na4Fe(CN) 6 20 Thus, the sodium of the neutralized sodium hydroxide is converted back to soluble sodium ferrocyanide which is the essential constituent of a starting solution fed into an electrolytic cell and which ferrocyanide may be eventually recycled thru the process by being returned to tank 10.
The hydroxide content of the neutralized sodium hydroxide is converted to ferric hydroxide which may be filtered out of the more or less neutralized liquor as by filter 18.
The amount of treating agent to be used depends upon the purity requirements of the final sodium ferricyanide product. In some instances, product containing a relatively small amount of sodium hydroxide may be unobjectionable, and in other instances it may be desired to form a product of best feasibly obtainable purity. The NaOH neutralization step of the present process is flexible and facilitates either substantially complete elimination of NaOH or removal of NaOH to any lesser extent desired. The amount of NaOH present in an anolyte liquor may be found by analyses and the theoretical quantity of treating agent needed to effect the desired degree of purification may be readily calculated. In usual practice of the process, I find that an ultimate ferricyanide product of acceptable purity with respect to NaOH content may be obtained when the quantity of treating agent employed is such as to lower pH of the treated anolyte solution to a value not higher than 10. In this situation, sodium ferricyanide product containing not more than about 0.2% NaOH may be obtained.
In the more satisfactory embodiments of the invention, it is preferred to control amount of treating agent used so that at the end of the treating step, pH of the anolyte solution is lowered to a value not in excess of 8.5. Ordinarily, it is preferred to use in excess of about 5 to 10% GO by weight of treating agent over the calculated theoretical requirements. Degree of neutralization, may be regulated by addition to the anolyte liquor being treated of successive increments of treating agent and by analyses along (5 toward the end point of neutralization. However, I find that the indicated pH control is the most satisfactory and practicable mode of adjusting neutralization and securing the soughtfor degree of purification. Generally speaking, in the case of use of Prussian blue as neutralizing agent satisfactory results are obtained when using about 2.4-2.5 parts by weight for every part of NaOH to be neutralized.
To further facilitate the neutralizing reaction in tank 1 and obtain a satisfactory filterable iron hydroxide precipitate, the treating operation should be carried out at temperatures from about 350 C. to not more than 500 C. Since the anolyte liquor discharged from cell 12 is ordinarily within this temperature range preliminary heating of the liquor in tank IS is unnecessary although this tank may be provided with a heating coil or other means suitable to keep the temperature during the neutralizing operation within the preferred 350 C.-500 C. range. The neutralizing agent is added to the liquor in tank 16 and the mass is agitated from say 10 to 20 minutes during which time the reaction of Equation (2) takes place and proceeds to completion. Thereafter, the liquor is filtered in filter 18 to remove the iron hydroxide which is discharged from the system at 19, the filtrate being run into a vacuum evaporator 2 1.
A representative neutralized and filtered solution as fed into evaporator 21 may contain from 300 to 350 g. p. 1. of Na3Fe(CN) 6, from 70 to 90 g. p. 1. of Na4Fe(CN)6, and from 0.1 to 0.5 g. p. 1. NaO'H.
In the particular example of operation of the process, the quantity of Prussian blue (containing 6% HO2) used to effect neutralization of NaOH in tank 16 is such as to lower the pH of the anolyte solution to between 8 and 8.5, and is approximately equivalent to 2.4 parts of Prussian blue per part of NaOH neutralized. The neutralized and filtered anolyte as fed into the evaporator 2 may analyze 324 g. p. 1. of Na3Fe(CN) 6, 78 g. p. 1. of Na4Fe(CN) 6 and 0.3 g. p. 1. of NaOH.
When Turnbull's blue or ferrous ferrocyanide are used as neutralizing agents, the reactions taking place are much the same as previously indicated and apparently proceed respectively as follows: (3) 6NaOH+Fe3(Fe(CN) 6)23Fe(OH) +2Na3Fe(CN) (4) 4NaO-H+Fe2Pe(CN) 62Fe (OH) 2+Nar4Pe (CN) 6 However, it is preferred to use Prussian blue as it has been found that the resulting iron hydroxide precipitate is more readily filterable. The next overall most satisfactory treating agent is Turnbull's blue.
The sodium ferricyanide filtrate of filter 19 contains in solution the sodium ferrocyanide which was not oxidized during electrolysis plus the sodium ferrocyanide formed in treating tank 16 by the reaction represented by Equation (2).
Another feature of the invention consists in the provision of procedure by which it is possible to remove, from the sodium ferricyanide solution, substantially all of such sodium ferrocyanide or of whatever lesser amount needs to be removed to form a ferricyanide product satisfying particular commercial requirements as to ferrocyanide impurity content.
While even in alkaline solution, solubility of sodium ferricyanide is high, I have observed that such solubility decreases appreciably in the presence of increasing amounts of sodium hydroxide, and that the presence of substantial quantity of sodium hydroxide in the sodium ferricyanide solution lowers the solubility of sodium ferricyanide and correspondingly enhances the difficulty of making an effective separation of ferricyanide and ferrocyanide. Hence, the neutralizing treatment in tank 16 affords not only removal from the system, as iron hydroxide, of hydroxide component of the sodium hydroxide but also the added important advantage of removal of sodium hydroxide from the solution, which correspondingly increases solubility of sodium ferricyanide and facilitates the subsequent separation of sodium ferricyanide and sodium ferrocyanide. Thus, in the following described operation for separation of ferrocyanide from ferricyanide, I ordinarily use a ferricyanide solution which has been neutralized to a pH value not exceeding 10 and preferably not exceeding 8.5.
In accordance with one important feature of the invention, I have found that sodium ferrocyanide contained in the filtrate of filter 18 may be separated from sodium ferricyanide by concentrating the filtrate, at temperature preferably less than that causing appreciable decomposition of ferricyanide, to a ferricyanide content such 13 that on cooling of the concentrated solution to about room temperature, substantially all or a desired lesser amount of the ferrocyanide crystallizes out in crystals of readily separable form.
My investigations show that a final sodium ferricyanide product of low ferrocyanide impurity content may be made by concentrating a solution such as a filtrate of filter 18 to a sodium ferricyanide content of not less than 450 g. p. 1. To prevent any substantial decomposition of sodium ferricyanide with the resultant formation of sodium ferrocyanide and iron hydroxide, the concentrating operation is carried out at temperature not in excess of about 800 C., such temperature being obtained by operating evaporator 21 under sufficiently reduced pressure, e. g., 28-29 inches of Hg. When proceeding under these conditions, there is formed a solution from which, on cooling to around room temperature, sodium ferrocyanide crystallizes out to such an extent that following separation of such ferrocyanide crystals there may be obtained a sodium ferricyanide solution in which the sodium ferrocyanide impurity content ordinarily does not exceed about 0.02%. In the better embodiments of the invention, concentration in evaporator 21 is preferably carried to a sodium ferricyanide content of not less than 500 g. p. 1., and where the commercial requirements are such as to call for a sodium ferricyanide product of best feasibly obtainable purity, evaporation is proceeded with to a sodium ferricyanide concentration of 550-575 g. p. 1. While any sodium ferricyanide precipitated during this concentration stage would not constitute a process loss, such sodium ferricyanide would have to be recycled. In order to avoid any appreciable formation of solid ferricyanide before separation of ferrocyanide, it is not desirable to concentrate in evaporator 2! to a ferricyanide content in excess of about 600 g. p. 1.
On completion of concentration, the evaporated liquor is run into a cooler and crystallizer 23 in which the solution is cooled down to about room temperature, e. g., 15-300 C. Such cooling crystallizes sodium ferrocyanide as Na4Fe(CN) 6.10H20 These crystals are separated out in filter 24 and are returned thru line 25 to. sodium ferrocyanide make-up solution in tank 10. According to this 0 procedure, the sodium ferrocyanide which is unoxidized in cell 12 and also the sodium ferrocyanide produced in tank 16 is recovered in a form which may be recycled thru the process, thus avoiding any loss of sodium or cyanide values A typical filtrate discharged from filter 24 may contain from 500 to 600 g. p. 1. of Na3Fe(CN)6, 0.2 to one g. p. 1. of Na4Fe(CN) 6, and 0.2 to 0 5 g. p. 1. of NaOH. In the illustrative example given, the solution in evaporator 21 is concentrated at temperature not -exceeding 700 C. under vacuum of about 28-29 inches of Hg to an Na3Fe(CN) concentration of about 550 g. p. 1., and the concentrated solution cooled to about 15" C. The filtrate of filter 24 may analyze 550 g. p. 1. of Na3Fe(CN)6, 0.2 g. p. 1. of Na4Fe(CN)6, and 0.3 g. p. 1. of NaOH. Although not essential, the sodium ferricyanide solution may be further filtered again in a clarifier 27 to produce in line 28 a crystal-clear solution. Part or all of this sodium ferricyanide solution, drawn off thru line 28, constitutes one of the products of the invention, and may be marketed at the sodium ferricyanide concentration thereof or diluted with water to reduce the sodium ferricyanide concentration, e. g., to a 40% solution of Na3Fe(CN)6.
If solid sodium ferricyanide product is desired, part or all of the clear solution is run thru line 29 into a second evaporator 30 in which the solution is further concentrated to a ferricyanide strength such that on cooling to say 15-30" C. a good crop of sodium ferricyanide crystals is obtained. Ordinarily, the solution is concentrated in evaporator 30 to a sodium ferricyanide content of about 650 g. p. 1. It is more important to prevent decomposition of sodium ferricyanide in evaporator 30 than in evaporator 21. While concenerating temperatures in evaporator 30 may be as high as 800 C., preferably concentrating temperatures should not exceed about 65-70" C.
The pressure during concentration in evaporator 30 may be about the same as in evaporator 21, e. g., 28-29 inches of Hg. On completion of concentration, the solution is run into the cooler and crystallizer, cooled down to about 15-30" C., and crystallized sodium ferricyanide is separated out in filter 33. Wet crystals, Na3Fe(CN) 6.2H20 are dried in a drier 35 at preferably maximum temperature of 60" C. The sodium ferricyanide solution filtrate of filter 33 is returned to evaporator 30. According to this mode of procedure, all of the sodium ferricyanide liquor run into evaporator 30 is eventually recovered as crystal product. In the particular example under consideration, in the evaporator 30, solution containing 550 g. p. 1. of Na3Fe(CN)6, 0.2 g. p. 1. of Na4Fe(CN)6 and 0.3 g. p. 1. of NaOH is evaporated to ferricyanide concentration of about 630 g. p. 1., and the final product comprises about 99.4% of the Na3Fe(CN)6.2H20 crystals, 0.3% of Na4Fe(CN)6, and 0.01% NaOH.
Preparation method of sodium ferrocyanide:
I claim: 1. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution with an iron salt, of an acid of the group consisting of ferro- and ferricyanic acids, reactable with sodium hydroxide to form a soluble sodium ironcyanide and to precipitate iron hydroxide, the quantity of said salt being such as to lower pH of the treated solution to a value not higher than 10, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 80" C. to a sodium ferricyanide content of not less than 450 g. p. 1., cooling the solution to crystallize sodium ferrocyanide, and separating sodium ferrocyanide crystals from the solution.
2. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution with an iron salt, of an acid of the group consisting of ferro- and ferricyanic acids, reactable with sodium hydroxide to form a soluble sodium iron-cyanide and to precipitate iron hydroxide, the quantity of said salt being such as to lower pH of the treated liquor to a value not higher than 10, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 800 C. to a sodium ferricyanide content of not less than 450 g. p. 1., 1I cooling the solution to crystallize sodium ferrocyanide, separating sodium ferrocyanide crystals from the solution, further concentrating the solution, and recovering solid sodium ferricyanide therefrom.
2o 3. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution .o with material of the group consisting of Prussian blue and Turnbull's blue, reactable with sodium hydroxide to form a soluble sodium iron-cyanide and to precipitate iron hydroxide, the quantity of said material being such as to lower pH of .o the treated liquor to a value not higher than 10, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 800 C. to a sodium ferricyanide content of not less than 450 g. p. 1., cooling the ::' solution to crystallize sodium ferrocyanide, and separating sodium ferrocyanide crystals from the solution.
4. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution with Prussian blue, reactable with sodium hydroxide to form sodium ferrocyanide and to precipitate iron hydroxide, the quantity of Prussian blue being such as to lower pH of the treated liquor to a value not higher than 10, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 800 C. to a sodium ferricyanide content of not less than 450 g. p. 1., cooling the solution to crystallize sodium ferrocyanide, separating sodium ferrocyanide crystals from the solution, further concentrating the solution, and recovering solid ferri;, cyanide therefrom.
5. In the process for making sodium ferricyanide by procedure involving electrolytic oxidation of a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodi6o um hydroxide and sodium ferrocyanide, and at least partial neutralization of sodium hydroxide, the steps comprising concentrating the solution, having pH value not higher than 10, at temperature not in excess of 800 C. to a sodium ferricyanide content of not less than 450 g. p. 1., cooling the solution to crystallize sodium ferrocyanide, and separating sodium ferrocyanide crystals from the solution.
6. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution with an iron salt, of an acid of the group consisting of ferro- and ferricyanic acids, reactable with sodium hydroxide to form a soluble sodium iron-cyanide and to precipitate iron hydroxide, the quantity of said salt being such as to lower pH of the treated solution to a value not higher than 8.5, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 800 C. to a sodium ferricyanide content of not less than 500 g. p. 1., cooling the solution to about room temperature to promote crystallization of sodium ferrocyanide, and separating sodium ferrocyanide crystals from the solution.
7. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution with an iron salt, of an acid of the group consisting of ferro- and ferricyanic acids, reactable with sodium hydroxide to form a soluble sodium iron-cyanide and to precipitate iron hydroxide, the quantity of said salt being such as to lower pH of the treated solution to a value not higher than 8.5, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 800 C. to a sodium ferricyanide content of not less than 500 g. p. 1., cooling the solution to about room temperature to promote crystallization of sodium ferrocyanide, separating sodium ferrocyanide crystals from the solution, further concentrating the solution, and recovering solid sodium ferricyanide therefrom.
8. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution with material of the group consisting of Prussian blue and Turnbull's blue, reactable with sodium hydroxide to form a soluble sodium ironcyanide and to precipitate iron hydroxide, the quantity of said material being such as to lower pH of the treated solution to a value not higher than 8.5, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 80° C. to a sodium ferricyanide content of not less than 500 g. p. 1. and not more than 600 g. p.
1., cooling the solution to about room temperature to promote crystallization of sodium ferrocyanide, and separating sodium ferrocyanide crystals from the solution.
9. The process for making sodium ferricyanide comprising electrolytically oxidizing a solution of sodium ferrocyanide to form a sodium ferricyanide solution containing sodium hydroxide and sodium ferrocyanide, treating the solution with Prussian blue, reactable with sodium hydroxide to form sodium ferrocyanide and to precipitate iron hydroxide, the quantity of Prussian blue being such as to lower pH of the treated solution to a value not higher than 8.5, separating iron hydroxide from the treated solution, concentrating the solution at temperature not in excess of 80° C. to a sodium ferricyanide content of not less than 500 g. p. 1. and not more than 600 g. p. 1., cooling the solution to about room temperature to promote crystallization of sodium ferrocyanide, separating sodium ferrocyanide crystals from the solution, further concentrating the solution, and recovering solid sodium ferricyanide therefrom.
You may like
Related articles And Qustion
Lastest Price from Sodium ferrocyanide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 13601-19-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $100.00-50.00/KG2024-10-11
- CAS:
- 13601-19-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg