Chemical properties
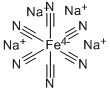
Sodium ferrocyanide is the sodium salt of the coordination compound of formula [Fe(CN)6]4−. In its hydrous form, Na4Fe(CN)6 • 10H2O (sodium ferrocyanide decahydrate), it is sometimes known as yellow prussiate of soda. It is a yellow crystalline solid that is soluble in water and insoluble in alcohol. The yellow color is the color of ferrocyanide anion. Despite the presence of the cyanide ligands, sodium ferrocyanide has low toxicity (acceptable daily intake 0–0.025 mg/kg body weight). The ferrocyanides are less toxic than many salts of cyanide, because they tend not to release free cyanide.However, like all ferrocyanide salt solutions, addition of an acid can result in the production of hydrogen cyanide gas, which is toxic.

Applications
Sodium hexacyanoferrate(II) decahydrate is being used and studied as a possible cathode material for sodium ion batteries.
Production
Sodium ferrocyanide is produced industrially from hydrogen cyanide, ferrous chloride, and calcium hydroxide, the combination of which affords Ca2[Fe(CN)6] • 11H2O. A solution of this salt is then treated with sodium salts to precipitate the mixed calcium-sodium salt CaNa2[Fe(CN)6], which in turn is treated with sodium carbonate to give the tetrasodium salt.
Storage
Hygroscopic, -20°C Freezer, Under inert atmosphere
Reactivity
Sodium ferrocyanide is a coordination compound of iron. The cyanide ligands are tightly bound to the iron, so it is not as toxic as simple inorganic cyanide salts. However, this compound can release hydrogen cyanide gas upon reaction with acids.
appearance
Sodium ferrocyanide is a yellow crystalline salt Na4Fe(CN)6 similar to potassium ferrocyanide and used in making iron blue pigments, blueprint paper, and dyes — Called also yellow prussiate of soda
Chemical Properties
solid
Uses
Sodium Ferrocyanide is an anticaking agent and crystallizing agent. It is sometimes added as a crystallizing agent to salt when it crystallizes to generate lagged and bulky crystals which resist caking. It also functions as a water-soluble anticaking agent.
Uses
Manufacture of sodium ferricyanide, blue pig-
ments, blueprint paper, anticaking agent for salt, ore
flotation, pickling metals, polymerization catalyst,
photographic fixing agent.
Definition
ChEBI: Sodium hexacyanoferrate(4-) is a hexacyanoferrate(4-) salt.
General Description
Odorless yellow solid. Sinks and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sodium ferrocyanide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Health Hazard
None recorded.
Flammability and Explosibility
Non flammable
reaction suitability
core: iron
reagent type: catalyst
Safety Profile
When heated to decomposition emits toxic fumes of CN-.
Purification Methods
Crystallise it from hot water (0.7mL/g), until free of ferricyanide as shown by the absence of formation of Prussian Blue colour with ferrous sulfate solution.