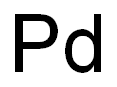Palladium Industrial applications and uses
Palladium (Pd), chemical element, the least dense and lowest-melting of the platinum metals of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table, used especially as a catalyst (a substance that speeds up chemical reactions without changing their products) and in alloys.
A precious gray-white metal, palladium is extremely ductile and easily worked. Palladium is not tarnished by the atmosphere at ordinary temperatures. Thus, the metal and its alloys serve as substitutes for platinum in jewelry and in electrical contacts; the beaten leaf is used for decorative purposes. Relatively small amounts of palladium alloyed with gold yield the best white gold. Palladium is used also in dental alloys. The chief use of palladium, however, is in automobile catalytic converters (often in combination with rhodium); the palladium serves as a catalyst to convert polluting hydrocarbons, carbon monoxide, and nitrogen oxide in the exhaust to water, carbon dioxide, and nitrogen. Palladium coatings, electrodeposited or chemically plated, have been used in printed-circuit components, and palladium is also used in multilayer ceramic capacitors.
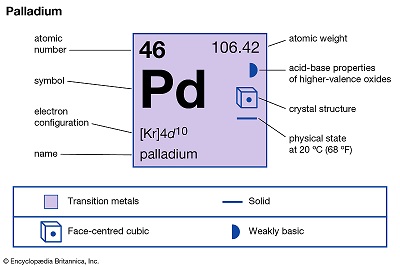
Native palladium, though rare, occurs alloyed with a little platinum and iridium in Colombia (department of Chocó), in Brazil (Itabira, Minas Gerais), in the Ural Mountains, and in South Africa (the Transvaal). Palladium is one of the most abundant platinum metals and occurs in Earth’s crust at an abundance of 0.015 part per million. For the mineralogical properties of palladium, see native element (table). Palladium also occurs alloyed with native platinum. It was first isolated (1803) from crude platinum by the English chemist and physicist William Hyde Wollaston. He named the element in honour of the newly discovered asteroid Pallas. Palladium is also associated with a number of gold, silver, copper and nickel ores. It is generally produced commercially as a by-product in the refining of copper and nickel ores. Russia, South Africa, Canada, and the United States were the world’s leading producers of palladium in the early 21st century.
Surfaces of palladium are excellent catalysts for chemical reactions involving hydrogen and oxygen, such as the hydrogenation of unsaturated organic compounds. Under suitable conditions (80 °C [176 °F] and 1 atmosphere), palladium absorbs more than 900 times its own volume of hydrogen. It expands and becomes harder, stronger, and less ductile in the process. The absorption also causes both the electrical conductivity and magnetic susceptibility to decrease. A metallic or alloylike hydride is formed from which the hydrogen can be removed by increased temperature and reduced pressure. Because hydrogen passes rapidly through the metal at high temperatures, heated palladium tubes impervious to other gases function as semipermeable membranes and are used to pass hydrogen in and out of closed gas systems or for hydrogen purification.
Description and general properties
Rhodium, chemical symbol Rh, atomic number 45, and relative atomic mass 102.90550(2), is a silvery-white, hard metal, fairly ductile when heated and having a high melting point (1966°C). It has a face-centered cubic (fcc) crystal lattice structure. Rhodium is corrosion resistant to most strong mineral acids including hot aqua regia up to 100°C. Moreover, it is also oxidation resistant in air at high temperatures, allowing solid rhodium or rhodium coatings to be used for high-temperature device manufacturing. However, upon heating it turns into an oxide when red and at higher temperatures turns back into the element. Moreover, it absorbs oxygen upon melting, releasing it upon solidification. Rhodium has the highest specular reflectivity, the whitest luster, and the highest electrical and thermal conductivities of the PGMs. Rhodium is a mononuclidic element with only one stable isotope, the nuclide 103Rh.

History
William Hyde Wollaston discovered rhodium in 1804 in crude platinum ore from South America soon after his discovery of another element, palladium. He dissolved the ore in aqua regia, neutralized the acid with sodium hydroxide (NaOH), and precipitated the platinum by treatment with salmiac (i.e., ammonium chloride, NH4Cl) as ammonium hexachloroplatinate (NH4PtCl6). Palladium was then removed as palladium cyanide by treatment with mercuric cyanide. The remaining material was a red material containing rhodium chloride salts from which rhodium metal was obtained by reduction with hydrogen gas.
Natural occurrence
Rhodium is one of the rarest element in the Earth’s crust with an abundance of 1 μg/kg (i.e., ppb wt.). Rhodium occurs in nature as a native metal along with other platinum-group metals in the native mineral iridosmine or in sulfide ores such as rhodite, sperrylite, and some copper-nickel ores.
Industrial applications and uses
Rhodium is a major component of industrial catalytic systems such as the BP-Monsanto process or as part of the catalytic system in car catalytic converters, used to clean up exhaust gases. It is also used as an alloying element to harden platinum and palladium alloys. Rhodium coating achieved by electroplating is used to protect silverware from tarnishing. Such alloys are used for furnace windings, thermocouple elements, bushings for glass fiber production, electrodes for aircraft spark plugs, and laboratory crucibles. In electrical engineering it is used as an electrical contact material as it has a low electrical resistance, a low and stable contact resistance, and high resistance to corrosion. Rhodium coatings produced by electroplating or vacuum evaporation are exceptionally hard and are used for optical instruments such as mirrors.
);You may like
Related articles And Qustion
See also
Lastest Price from Palladium manufacturers

US $1.00/g2024-04-15
- CAS:
- 7440-05-3
- Min. Order:
- 10g
- Purity:
- 99
- Supply Ability:
- 20ton
US $8.00-0.80/KG2024-04-09
- CAS:
- 7440-05-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available