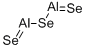Crystal Structure of Aluminum Selenide
Aluminum selenide, an inorganic compound, functions as a semiconductor with an optimal pH of 9.5. Its preparation involves mixing a solution of aluminum and hydrochloric acid with a solution of sodium hydroxide. Additionally, aluminum selenide exhibits efficient light absorption, effectively converting light into heat energy. Notably, this material boasts homogeneous optical properties, ensuring uniformity throughout. Moreover, aluminum selenide serves as a catalyst in various reactions, including the conversion of chloride to chlorine gas and carbon dioxide to methane gas.
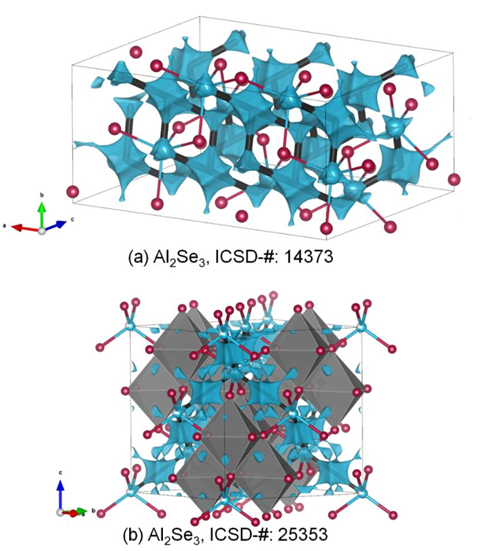
Figure 1. Crystal structure of aluminum selenide. Blue spheres: Al, red spheres: Se, grey polyhedra: Al-Se, black rods: conduction path identified by VDP, blue isosurface: percolating Al3+ -migration energy structure from BVSE.[1]
As shows in above figure, aluminum selenide (Al2Se3) crystallizes in the monoclinic Cc space group. The structure is three-dimensional. there are two inequivalent Al3+ sites. In the first Al3+ site, Al3+ is bonded to four Se2- atoms to form corner-sharing AlSe4 tetrahedra. There are a spread of Al–Se bond distances ranging from 2.33–2.47 Å. In the second Al3+ site, Al3+ is bonded to four Se2- atoms to form corner-sharing AlSe4 tetrahedra. There are a spread of Al–Se bond distances ranging from 2.34–2.48 Å. There are three inequivalent Se2- sites. In the first Se2- site, Se2- is bonded in a water-like geometry to two Al3+ atoms. In the second Se2- site, Se2- is bonded in a trigonal non-coplanar geometry to three Al3+ atoms. In the third Se2- site, Se2- is bonded in a trigonal non-coplanar geometry to three Al3+ atoms[2].
Reference
[1] Sulphur- and selenium-containing compounds potentially exhibiting Al-ion conductivity. Chem. Eur. J. DOI:10.1002/chem.201901438
[2] G.A. Steigmann and J. Goodyear. The crystal structure of al2 se3.Acta Crystallographica (1,1948-23,1967), 20:617–619, 1966.
You may like
See also
Lastest Price from ALUMINUM SELENIDE manufacturers
US $0.00-0.00/KG2024-08-17
- CAS:
- 1302-82-5
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KG