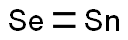Crystal Structure and Property of Tin Selenide
The indirect bandgap semiconductor tin selenide (SnSe) has been a research hotspot in the thermoelectric fields since a ZT (figure of merit) value of 2.6 at 923 K in SnSe single crystals along the b‐axis is reported. SnSe has also been extensively studied in the photovoltaic (PV) application for its extraordinary advantages including excellent optoelectronic properties, absence of toxicity, cheap raw materials, and relative abundance. Moreover, the thermoelectric and optoelectronic properties of SnSe can be regulated by the structural transformation and appropriate doping. This article will introduce the crystal structure of tin selenide[1].
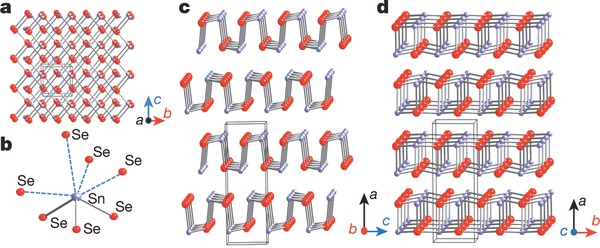
Figure 1. Crystal structure of tin selenide[1].
SnSe bulk structure demonstrates a layered orthorhombic structure having space group Pnma. The structure consists of strongly bound double layers and can be regarded as a distorted rocksalt phase. Moreover, its perspective views are different along the a, b, and c axial directions, which lead to its anisotropic nature.
There are SnSe slabs with a thickness of nearly two‐atoms with strong Sn—Se bonds within the plane of slabs, i.e., along b–c plane and weaker along a‐axis. The perspective view along the b‐axis is a zigzag accordion like projection but along the c‐axis is an armchair form. Scientists have detected high ZT values along the b‐axis near and above the transition temperature of about 800 K at which the structure converts from space group of Pnma to Cmcm, with the bandgap changing from 0.61 to 0.39 eV, and the lattice parameters changing from a = 11.49 Å, b = 4.44 Å, c = 4.135 Å to a = 4.31 Å, b = 11.70 Å, c = 4.31 Å. The highly distorted SnSe7 coordination polyhedral contained in SnSe crystal has three short and four long Sn—Se bonds, as shown in Figure 1: a) Crystal structure along the a‐axis: grey, Sn atoms; red, Se atoms. b) Highly distorted SnSe7 coordination polyhedron with three short and four long Sn—Se bonds. c) Structure along the b‐axis. d) Structure along the c‐axis[1].
Reference
[1] Tin Selenide (SnSe): Growth, Properties, and Applications. Adv Sci (Weinh). 2018 Apr; 5(4): 1700602.
doi: 10.1002/advs.201700602
You may like
See also
Lastest Price from TIN SELENIDE manufacturers
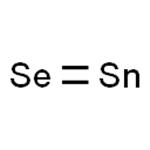
US $0.00-0.00/KG2024-09-04
- CAS:
- 1315-06-6
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KG