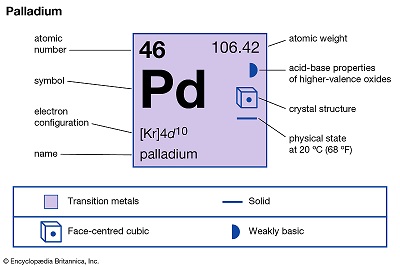
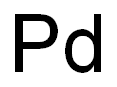Palladium-Hazard and Toxicity
Description
Palladium was discovered in 1803 by W.H. Wollaston during refining and purification of- platinum metal. This new metal was found in the aqua regia extract of native platinum and was detected in solution after platinum was precipitated. It was removed as ammonium chloroplatinate. Treating this solution with mercurous cyanide precipitated a yellow palladium complex salt. The precipitate was washed and ignited to form palladium metal. Wollaston named the element palladium after the newly discovered asteroid Pallas.

Palladium in nature is always associated with other platinum group metals. Its abundance in earth's crust is estimated at 0.015 mg/kg, about three times more abundant than platinum. Palladium is used mostly in alloys and the majority of its alloys are used for electronics and telecommunications. They are contacts in electrical relays and automatic switching gear.
Palladium-gold alloys are applied widely in dentistry and medicine. They are in devices for replacement of damaged bones and joints and as support in porcelain-overlay bridgework. Palladium alloys are used in decoration and jewelry as a substitute for gold. They are used in gems, watch cases and brooches.
Toxicity Data
LD50 oral (rat) 200 mg/kg (palladium chloride)
LC50 intratracheal (rat) 6 mg/kg (palladium chloride)
Major Hazards
May ignite on exposure to air, particularly when containing adsorbed hydrogen; readily causes ignition of flammable solvents in the presence of air. Palladium is not combustible except as fine powder or dust. Several of palladium’s compoundsare oxidizing agents, and some react violently with organic substances.
Palladium is a metal with low toxicity as conventionally measured (eg. Ld50). Recent research on the mechanism of palladium toxicity suggests high toxicity if measured on a longer timeframe and at the cellular level in the liver and kidney. Mitochondria appear to have a key role in palladium toxicity via mitochondrial membrane potential collapse and depletion of the cellular glutathione (GSH) level.
Until that recent work, it had been thought that palladium was poorly absorbed by the human body when ingested. Plants such as the water hyacinth are killed by low levels of palladium salts, but most other plants tolerate it, although tests show that, at levels above 0.0003%, growth is affected. High doses of palladium could be poisonous; tests on rodents suggest it may be carcinogenic, though until the recent research cited above, no clear evidence indicated that the element harms humans.
Toxicity
Very little information is available on the toxicity of palladium and its compounds. There is some evidence that chronic exposure to palladium particles in dust can have toxic effects on the blood and respiratory systems. Finely divided carbon is irritating to mucous membranes and the upper respiratory tract.
Flammability and Explosibility
Palladium on carbon catalysts containing adsorbed hydrogen are pyrophoric, particularly when dry and at elevated temperatures. Palladium on carbon catalysts prepared by formaldehyde reduction are less pyrophoric than those reduced with hydrogen. Finely divided carbon, like most materials in powder form, is capable of creating a dust explosion.
Reactivity and Incompatibility
Catalysts prepared on high surface area supports are highly active and readily cause ignition of hydrogen/air and solvent/air mixtures. Methanol is notable for easy ignition because of its high volatility. Addition of catalyst to a tetrahydroborate solution may cause ignition of liberated hydrogen.
Storage and Handling
In particular, palladium on carbon should always be handled under an inert atmosphere (preferably argon), and reaction vessels should be flushed with inert gas before the catalyst is added. Dry catalyst should never be added to an organic solvent in the presence of air. Palladium on carbon recovered from catalytic hydrogenation reactions by filtration requires careful handling because it is usually saturated with hydrogen and will ignite spontaneously on exposure to air. The filter cake should never be allowed to dry, and the moist material should be added to a large quantity of water and disposed of properly.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If palladium on carbon is ingested, obtain medical attention immediately.
If large amounts of dust are inhaled, move the person to fresh air and seek medical attention at once. In the event of a spill, remove all ignition sources, wet the palladium on carbon with water, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large release in a confined area.
Disposal
Excess palladium on carbon and waste material containing this substance should be covered in water, placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
);You may like
Related articles And Qustion
See also
Lastest Price from Palladium manufacturers

US $1.00/g2024-04-15
- CAS:
- 7440-05-3
- Min. Order:
- 10g
- Purity:
- 99
- Supply Ability:
- 20ton
US $8.00-0.80/KG2024-04-09
- CAS:
- 7440-05-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available