General Properties of Epichlorohydrin
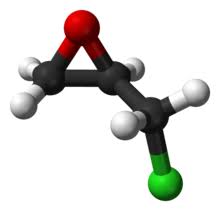
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, and elastomers.
Uses
Epichlorohydrin (ECH or EPI) is a highly reactive chemical intermediate.In its pure form, it is a clear, colorless liquid. The presence of both an epoxide ring and a chlorine atom in the molecule allows epichlorohydrin to readily undergo a variety of chemical reactions with many types of compounds.This versatility earns its wide use as a chemical intermediate.
Toxcity
Epichlorohydrin is flammable and considered as a hazardous chemicalIf released into the environment, however, it biodegrades rapidlyDirect, prolonged contact with pure Epichlorohydrin as liquid can severely damage the skin and eyes. Vapors may also produce eye irritation and damage to cornea of the eye.
Epichlorohydrin is a probable carcinogen, a poison that can cause death at higher exposures, a mutagen and a skin sensitizer.Occupational exposures are possible; however, consumer exposures are not likely because end-use products are expected to contain only trace levels of Epichlorohydrin.
Industrial Production
Industrial operations use Epichlorohydrin in closed systems and are designed with engineering controls to minimize personnel and environmental exposures.
The primary process for producing Epichlorohydrin consists of three steps:
1. Chlorination of propylene to form allyl chloride
2. Reaction of the allyl chloride with hypochlorous acid to produce glycerol dichlorohydrin
3. Reaction of the glycerol dichlorohydrin isomers with sodium hydroxide or calcium hydroxide to produce Epichlorohydrin.
Epichlorohydrin is produced out of propylene or natural glycerin. The new processes developped to use glycerin offer the advantage to be more energy efficient and to operate with a natural renewable feedstock. Approximately 76% of the world’s consumption of (EPI) is used to make epoxy resins.
);You may like
Related articles And Qustion
Lastest Price from Epichlorohydrin manufacturers
US $50.00-1.00/KG2024-03-25
- CAS:
- 106-89-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $3.00/L2024-02-27
- CAS:
- 106-89-8
- Min. Order:
- 1L
- Purity:
- 99.9%
- Supply Ability:
- 3000MT/MONTH