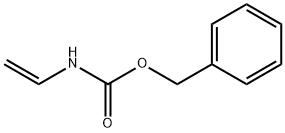
How to synthesize Benzyl vinylcarbamate?
Govindan developed an improved method that can be easily scaled up and has been designed to prepare benzyl-N-vinyl carbamate. In this method, vinyl isocyanate formed by the Curtius rearrangement of acryloyl azide is codistilled with a solvent such as toluene into benzyl alcohol containing a catalyst and inhibitor. The product thus obtained can be purified by crystallization, avoiding purification by high-vacuum distillation or chromatography.
Synthesis of Acryloyl Azide
A 1-L reactor was charged with 68.4 g (1.05 mol) of sodium azide, 200 mL of water, 200 mL of toluene, and 0.09 g of Adogen 464 (methyltrialkylammonium chloride). The mixture was cooled by stirring in an ice-water bath, and 90 g (1 mol) of acryloyl chloride was added dropwise over a period of 1.5 h at 0-5 °C. After the addition, the mixture was stirred for 45 min. The organic phase was separated and stored at 0-5 °C until used.
Synthesis of Benzyl vinylcarbamate
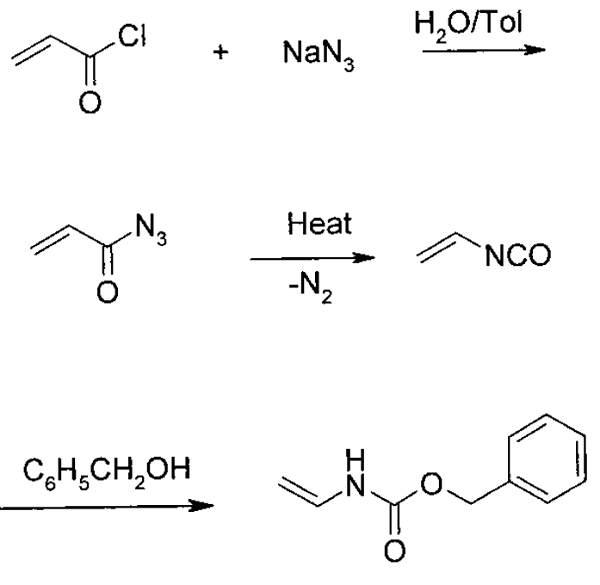
The flask was charged 150- 200 mL of toluene and 0.5 g of phenothiazine. The toluene solution was heated to 105-110 °C. The receiver was charged with 86 g (0.8 mol) of benzyl alcohol, 0.05 g of phenothiazine, and 0.1-0.3 g of triethylamine. This mixture was cooled in ice and stirred. A solution of acryloyl azide (1 mol) prepared as described before was pumped into the distillation flask over a period of 4-5 h, maintaining a pot temperature at 105-110 °C with a heating mantle. The vapor temperature varied, depending on the rate of addition of the azide, but was in the range of 80-100 °C. The distillate was collected directly in the benzyl alcohol mixture. After adding acryloyl azide, the distillation continued to distill out 10-20 mL of toluene. The receiver was then isolated from the distillation setup, and its contents were stirred at 0-5 °C for 1-2 h. The product mixture was then allowed to warm gradually to room temperature and stirred until HPLC analysis indicated a complete reaction. The mixture was then stripped in vacuo to a weight of 200-250 g. The residue was added to 300-350 mL of heptane and cooled by stirring to 15 °C. A few seed crystals of Benzyl vinylcarbamate were added, and the mixture was stirred for 2-3 h. Benzyl vinylcarbamate was filtered, washed with heptane, and dried in vacuo. Yield 115-128 g (65-72%).
References
[1] Govindan, Cheruthur K. “An Improved Process for the Preparation of Benzyl-N-vinyl Carbamate1.” Organic Process Research Development 6 1 (2001): 74–77.