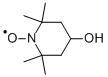
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy- Reaction / Application on synthetic works
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy is a heterocyclic compound, which is a 4-substituted 2,2,6,6-tetramethylpiperidyl-1-oxy (TEMPO) derivative. Like the related TEMPO, it is used as a catalyst and chemical oxidant by virtue of being a stable radical. It is a low-molecular weight compound, and its major appeal over TEMPO is that is less expensive, being produced from triacetone amine, which is itself made via the condensation of acetone and ammonia. This makes it economically viable on an industrial scale.
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy may be employed as catalyst for the oxidation of alcohols to the corresponding aldehydes.[1] It may be employed for the preparation of TEMPO based polymer catalyst systems, which are useful for the Anelli oxidation of various primary alcohols.[2] It may be used as starting reagent in the synthesis of 4-(2,2,6,6-tetramethyl-1-oxyl-4-piperidoxyl)butyl bromide.[3]
A fluorous-tagged TEMPO derivative was prepared from the derived TEMPO propargylic ether and subsequent "click" reaction with a fluorous azide. This TEMPO derivative proved to be a highly effective catalyst in the oxidation of alcohols with bleach.[4]
In biochemical research, it has been investigated as an agent for limiting reactive oxygen species. It catalyzes the disproportionation of superoxide, facilitates hydrogen peroxide metabolism, and inhibits Fenton chemistry. Spin label reagent used in the characterization of antagonist and agonist binding sites of NK1 receptor. Also used to measure reactive oxygen generation in heart muscle by electron spin resonance spectroscopy. Cells that overexpress CYP2E1 can be protected from arachidonic acid toxicity damage by TEMPOL. [5]
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy can also be used as an important organic intermediate to synthetize its substituted products.
Example 1
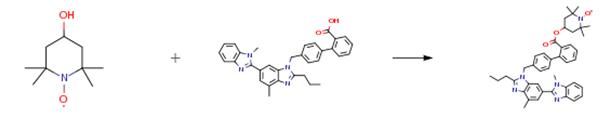
To an ice bath cooled solution of telmisartan (0.8 g, 1.55 mmol) in DMF (50 mL) was added 1-hydroxybenzotriazole (HOBt, 0.25 g, 1.87 mmol), 4-dimethylamino pyridine (DMAP, 0.23 g, 1.87 mmol), and l-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 0.39 g, 2.02 mmol), followed by tempol (0.29 g, 1.712 mmol). The mixture was stirred at room temperature for 48 h. Water (15 mL) was added to the mixture and stirred at room temperature for 10 minutes. The mixture was extracted with ethyl Atty Docket No. GUX-024.25 acetate (3 x l5 mL). The organic layer was washed with sat. LiCl (15 mL), sat. NaHC03 (15 mL), water (15 mL) and brine (15 mL), dried over Na2S04. The solvent was evaporated and the residue was purified by flash chromatography using CH2Cl2-MeOH to afford YK-4-250 as a pink soft solid (0.81 g, 78percent).
Example 2
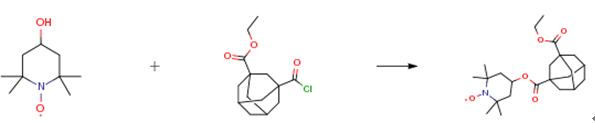
To a solution of staring material (2.70 g, 11 mmol) in THF (20 mL) was added thionyl chloride (3.6 mL, 50 mmol) and two drops of N,N-dimethylformamide as catalyst. The reaction mixture was refluxed for 3 h and solvents were evaporated. Subsequently theacyl chloride intermediate was added to a stirred solution of 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (Tempol, 2.07 g,12 mmol) and triethylamine (1.6 mL, 12 mmol) in toluene (50 mL).The resulting mixture was stirred for 8 h at 70 °C. After consumption of the starting material the mixture was washed with water(6×50 mL), dried over MgSO4, concentrated. The crude material was purified by flash column chromatography (petroleum ether/EtOAc) to yield the title compound as an orange solid 1.36 g. M.p.56°C;
Example 3
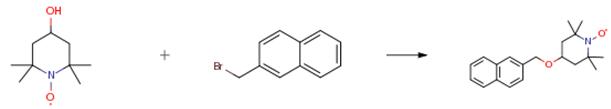
4-hydroxy TEMPO (1.72 g, 10 mmol) was dissolved in 10 ml of dry THF and cooled to 0 °C. Sodium hydride (0.29 g, 12 mmol)was added gradually with constant stirring. The reaction was stirred for 30 min at low temperature, followed by addition of 2-bromo methylnaphthalene (2.2 g, 10mmol). The reaction mixture was then warmed to room temperature and stirred until completion (1 day), as monitored by TLC. The reaction mixture was diluted with water (30 ml). The resulting solution was extracted with ethyl acetate (3×50 ml). The combined organic layers were dried with anhydrous sodium sulphate (15 g), filtered, concentrated and purified by column chromatography using 0 to 10 percent ethyl acetate/hexane as the eluent to provide an orange solid powder.
References
1. Fritz-Langhals E. Technical production of aldehydes by continuous bleach oxidation of alcohols catalyzed by 4-hydroxy-TEMPO[J]. Organic Process Research and Development, 2005, 9(5):577-582.
2. Tanyeli C, Gumus A. Synthesis of polymer-supported TEMPO catalysts and their application in the oxidation of various alcohols[J]. Tetrahedron Letters, 2003, 44(8):1639-1642.
3. Qian W, et al. Clean and selective oxidation of alcohols catalyzed by ion-supported TEMPO in water[J]. Tetrahedron, 2006, 62(4):556-562.
4. Gheorghe A, Cuevas-Yañez E, Horn J, Bannwarth W, Narsaiah B, Reiser OA .Facile Strategy to a New Fluorous-Tagged, Immobilized TEMPO Catalyst Using a Click Reaction, and Its Catalytic Activity[J]. Synlett, 2006: 2767–2770
5. Sledziński Z, et. al. Protective effect of 4-hydroxy-TEMPO, a low molecular weight superoxide dismutase mimic, on free radical toxicity in experimental pancreatitis[J]. International journal of pancreatology, 1995, 18(2):153–16
You may like
Related articles And Qustion
See also
Lastest Price from 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy manufacturers

US $1.00/KG2025-04-21
- CAS:
- 2226-96-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $3.00/kg2025-04-21
- CAS:
- 2226-96-2
- Min. Order:
- 1 mkg
- Purity:
- 99.5%
- Supply Ability:
- 500mt