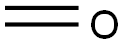Formaldehyde-Hazard and Toxicity
Description
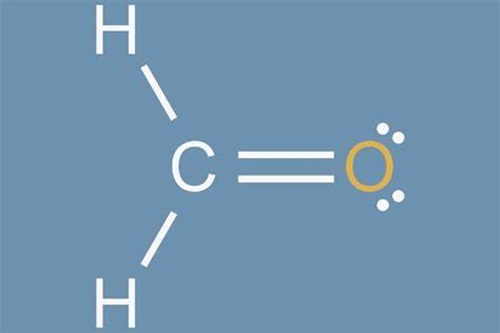
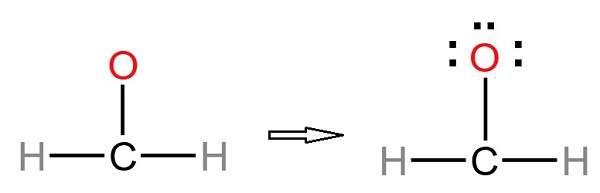
Formaldehyde, also called formic aldehyde or methyl aldehyde, has extensive application. For instance, it is used as a tissue preservative or organic chemical reagent. Thus, formaldehyde is very common to the chemical industry. In fact, formaldehyde is an important chemical used widely by industry to manufacture building materials and numerous household products. It is also a by-product of combustion and certain other natural processes. It is present in substantial concentrations both indoors and outdoors.

Formaldehyde is well known as a preservative in medical laboratories, as an embalming fluid, and as a steriliser. Its primary use is in the production of resins and as a chemical intermediate. Urea–formaldehyde (uf) and phenol–formaldehyde (pf) resins are used in foam insulations, as adhesives in the production of particle board and plywood, and in the treating of textiles.
Sources of formaldehyde in the home include building materials, smoking, household products, and the use of unvented, fuel-burning appliances, like gas stoves or kerosene space heaters. Formaldehyde, by itself or in combination with other chemicals, serves a number of purposes in manufactured products.
Toxicity Data
LD50 oral (rat) 500 mg/kg
LD50 skin (rabbit) 270 mg/kg
LC50 inhal (rat) 203 mg/m3 (2 h)
PEL (OSHA) 1 ppm (1.5 mg/m3)
TLV-TWA (ACGIH) 0.3 ppm (ceiling)(0.37 mg/m3)
STEL (OSHA) 2 ppm (2.5 mg/m3)
Major Hazards
Probable human carcinogen (OSHA "select carcinogen"); moderate acute toxicity; skin sensitizer. Formaldehyde is a colorless, pungent-smelling gas. Exposures to low levels of formaldehyde cause irritation of the eyes, nose, throat, skin, nausea, and diffi culty in breathing. Short-term exposure to formaldehyde can be fatal. Long-term exposure to low levels of formaldehyde may cause respiratory diffi culty, eczema, and sensitization. Occupational workers with asthma have been found to be more sensitive to the effects of inhaled formaldehyde; in high concentrations, formaldehyde triggers attacks in people with asthma.
Toxicity
Formaldehyde is moderately toxic by skin contact and inhalation. Exposure to formaldehyde gas can cause irritation of the eyes and respiratory tract, coughing, dry throat, tightening of the chest, headache, a sensation of pressure in the head, and palpitations of the heart. Exposure to 0.1 to 5 ppm causes irritation of the eyes, nose, and throat; above 10 ppm severe lacrimation occurs, burning in the nose and throat is experienced, and breathing becomes difficult.
Acute exposure to concentrations above 25 ppm can cause serious injury, including fatal pulmonary edema. Formaldehyde has low acute toxicity via the oral route. Ingestion can cause irritation of the mouth, throat, and stomach, nausea, vomiting, convulsions, and coma. An oral dose of 30 to 100 mL of 37% formalin can be fatal in humans. Formalin solutions can cause severe eye burns and loss of vision. Eye contact may lead to delayed effects that are not appreciably eased by eye washing.
Toxicity
Formaldehyde is regulated by OSHA as a carcinogen (Standard 1910.1048) and is listed in IARC Group 2A ("probable human carcinogen"). This substance is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard. Prolonged or repeated exposure to formaldehyde can cause dermatitis and sensitization of the skin and respiratory tract. Following skin contact, a symptomfree period may occur in sensitized individuals. Subsequent exposures can then lead to itching, redness, and the formation of blisters.
Flammability and Explosibility
Formaldehyde gas is extremely flammable; formalin solution is a combustible liquid (NFPA rating = 2 for 37% formaldehyde (15% methanol), NFPA rating = 4 for 37% formaldehyde (methanol free)). Toxic vapors may be given off in a fire. Carbon dioxide or dry chemical extinguishers should be used to fight formaldehyde fires.
Reactivity and Incompatibility
Formaldehyde may react violently with strong oxidizing agents, ammonia and strong alkalis, isocyanates, peracids, anhydrides, and inorganic acids. Formaldehyde reacts with HCl to form the potent carcinogen, bis-chloromethyl ether.
Storage and Handling
In particular, work with formaldehyde should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Formaldehyde should be used only in areas free of ignition sources. Containers of formaldehyde should be stored in secondary containers in areas separate from oxidizers and bases.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If formaldehyde is ingested, obtain medical attention immediately.
If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once. In the event of a spill, remove all ignition sources, soak up the formaldehyde with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Excess formaldehyde and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
You may like
Related articles And Qustion
See also
Lastest Price from Formaldehyde manufacturers

US $10.00/kg2025-04-21
- CAS:
- 50-00-0
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100

US $450.00-350.00/T2025-03-21
- CAS:
- 50-00-0
- Min. Order:
- 20T
- Purity:
- 37% 40%
- Supply Ability:
- 1000 Tons per year