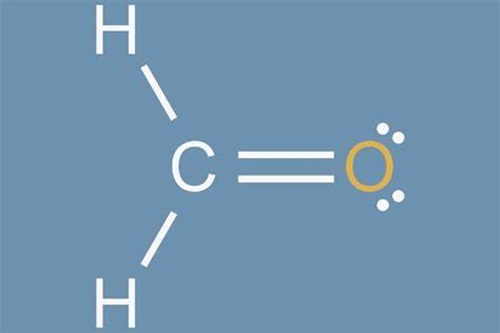
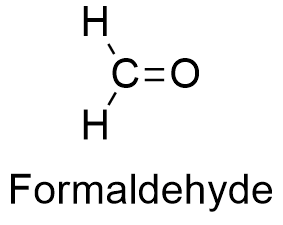
What is the Lewis structure for Formaldehyde?
The carcinogen Formaldehyde
Formaldehyde, symbolized as CH2O, is a simple and widespread organic compound. This colorless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Due to its preservative and disinfectant properties, Formaldehyde is often applied in the industrial production of different products, such as textiles, insulation materials, or cosmetics. However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there are numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Understanding the structure of Formaldehyde is crucial in comprehending its chemical properties and reactions[1].
There are 6 steps to draw the Lewis structure of Formaldehyde.
Step 1: Determine the total number of valence electrons in the Formaldehyde. Hydrogen, a Group IA element, has one electron in its outer shell. Oxygen, a Group VIA element, has six electrons in its outer shell. Carbon is a Group IVA element with four electrons in its outer shell. Hence, the total valence electrons available for the Lewis structure of Formaldehyde = 4 *1 + 1*2 + 6*1 = 12
Step 2: Calculate the number of electron pairs (lone pairs and bonds). Total valence electron pairs = σ bonds + π bonds + lone pairs at valence shells.
Total electron pairs are calculated by dividing the total valence electron count by two. In the valence shells of the HCHO molecule, there are 6 pairs of electrons.
Step 3: Choosing the central atom. A hydrogen atom cannot be a center atom because a hydrogen atom cannot make more than 1 bond. Carbon is more electropositive than oxygen. Therefore, it will be the central atom. After that, connect the outer atoms to the central atom with a single bond (shown below).
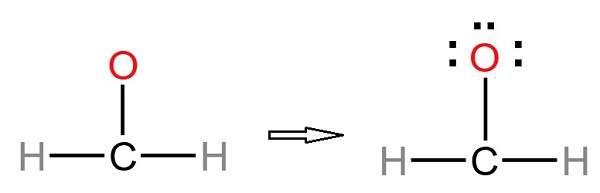
Step 4: Mark atoms with lone pairs. As shown above, there are already three bonds, and the other three lone pairs should be marked on hydrogen, carbon, and oxygen atoms. First, mark any remaining lone pairs on the outside atoms. The remaining three lone pairs on the oxygen atom should be marked. The charges on the oxygen and central carbon atoms are as follows.
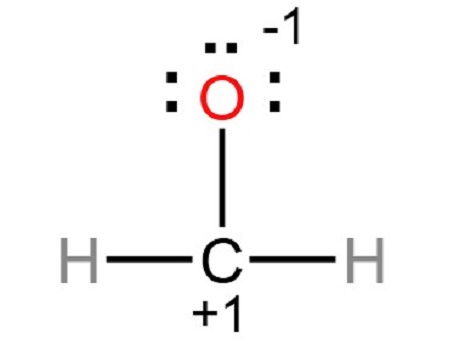
Step 5: In order to obtain the best Lewis structure, minimize charges on atoms by converting lone pairs to bonds. There are charges on the carbon and oxygen atoms in the center. Hence, a single lone pair of oxygen atoms should be converted to a single covalent bond.
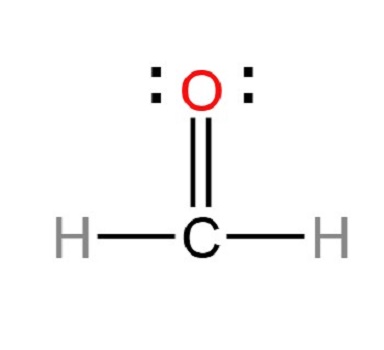
Step 6: Check the stability of the structure
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
For hydrogen atom: Formal charge=1-0-2/2=0
For carbon atom: Formal charge=4-0-8/2=0
For oxygen atom: Formal charge=6-4-4/2=0
Since the overall formal charge is zero, the above Lewis structure of Formaldehyde is the most appropriate, reliable, and stable.
Reference
[1] Francisco, BrandO Pedro , R. R. Miguel , and Rodrigues José António. "GDME-based methodology for the determination of free formaldehyde in cosmetics and hygiene products containing formaldehyde releasers."Analytical and Bioanalytical Chemistry 410(2018):6873-6880.
You may like
Related articles And Qustion
Lastest Price from Formaldehyde manufacturers

US $10.00/kg2025-04-21
- CAS:
- 50-00-0
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100

US $450.00-350.00/T2025-03-21
- CAS:
- 50-00-0
- Min. Order:
- 20T
- Purity:
- 37% 40%
- Supply Ability:
- 1000 Tons per year