Coumarins — An Important Class of Phytochemicals
1. Introduction
Phytochemicals are chemical compounds that occur naturally in the plant kingdom. Some are responsible for the organoleptic properties of the natural sources in which they are present. The term is generally used to refer to those chemicals that may have biological significance, for example carotenoids, flavonoids, coumarins, or chromones, but not all are established as essential nutrients. There may be as many as 4,000 different phytochemicals having potential activity against several diseases such as cancer and metabolic or degenerative diseases.
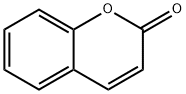
Among them, coumarins are a family of benzopyrones (1,2-benzopyrones or 2H-1-benzopyran-2-ones) widely distributed in the nature. They represent an important family of naturally occurring and/or synthetic oxygen-containing heterocycles, bearing a typical benzopyrone framework.
The name coumarin comes from a French term for the Tonka bean, coumarou, seeds of Dipteryx odorata (Coumarouna odorata) (Fabaceae/Leguminosae), one of the sources from which coumarin was first isolated as a natural product in 1820. It has a sweet odor, easy to be recognized as the scent of new-mown hay; because of that, coumarin has been used in perfumes since 1882. It is presumed to be produced by plants as a chemical defense to discourage predation [2, 3].

Coumarinic compounds are a class of lactones structurally constructed by a benzene ring fused to α-pyrone ring, and essentially possess - conjugated system with rich electron and good charge-transport properties [4, 5]. The simplicity and versatility of the coumarin scaffold make it an interesting starting-point for a wide range of applications [6-8]. There are coumarins as perfumes, cosmetics, and industrial additives. Some of its derivatives have been used as aroma enhancers in tobaccos and certain alcoholic drinks [9, 10]. But their most relevant role is described in natural products, organic chemistry, and medicinal chemistry [11, 12]. The extraction, synthesis, and evaluation of coumarins have become an extremely attractive and rapidly developing topic [13, 14]. Moreover, a lot of coumarin compounds as medicinal candidates for drugs with strong pharmacological activity, low toxicity and side effects, fewer drug resistance, high bioavailability, broad spectrum, better curative effects, etc., to treat various types of diseases are being actively studied [15]. Several efforts have been made mainly in developing coumarin-based anticoagulant, antioxidant [16], antimicrobial (anti-viral, antifungal, and anti-parasitic) [10, 17], anticancer [18-20], anti-diabetic, analgesic, anti-neurodegenerative, and anti-inflammatory agents [10, 21]. Moreover, the unique and versatile oxygen-containing heterocyclic structure makes coumarin compounds occupy an important place in medicinal chemistry [22, 23]. In addition, studies have been done regarding coumarins as bioactive agents [24], as well as supramolecular medicinal drugs, diagnostic agents and pathologic probes, and biological stains [25]. Particularly, the large - conjugated system in the coumarinic ring, with electron-rich and charge-transport properties, is important in the interaction of this scaffold with molecules and ions. Coumarin-based ion receptors, fluorescent probes, and biological stains are growing quickly and have extensive applications to monitor timely enzyme activity, complex biological events, as well as accurate pharmacological and pharmacokinetic properties in living cells [26, 27].
Coumarin was first synthesized in 1868, and it was used in the pharmaceutical industry as a precursor in the synthesis of a number of synthetic anticoagulant pharmaceuticals, starting with dicoumarol (removed from the current therapy) [28]. So far, some interesting coumarin-based anticoagulant drugs have extensively been used in clinics [29]. Coumarins are a type of vitamin K antagonists [30]. The most notable ones are warfarin, acenocumarol, and phenprocoumon, currently in use in several countries [31, 32]. Warfarin is employed more frequently than acenocoumarol because of its longer half-life (36 h), theoretically providing more stable anticoagulation and avoiding factor VII fluctuations that potentially occur during acenocoumarol treatment (half-life 10 h) [33]. Nowadays, some coumarins proved to be enzymatic inhibitory agents [monoamine oxidase (MAO) inhibitors, acetylcholinesterase (AChE) inhibitors, and butyrylcholinesterase (BuChE) inhibitors] with great potential in neurodegenerative diseases (ND) [34-38]. These studies represent an important tendency in the coumarin’s chemistry and biological evaluation [39-41].
Therefore, the coumarin ring is prevalently applied to construct several functional molecules in the medicinal field. A great deal of work has been done directed towards the separation and purification of naturally occurring biological coumarins from a variety of plants, animals, and microorganisms, as well as towards the artificial synthesis of coumarin compounds with novel structures and properties [42]. Coumarin compounds as medicinal drugs have been increasingly attracting special interest due to their underlying outstanding contributions in the prevention and treatment of diseases, and the related researches and developments have become an extremely attractive highlighted area.
In this context, an overview of the role of coumarins as important phytochemicals and their interesting applications will be presented and discussed. The origin, natural sources, biosynthesis, and applications are going to be presented in this chapter.
2. Natural occurring coumarins
Coumarin (Figure 1) and its derivatives are an important group of natural compounds widely distributed in the natural kingdom [43]. They can be found in the integument of seeds, fruits, flowers, roots, leaves, and stems, although the largest concentration is generally in fruits and flowers [44]. Originally, coumarin was isolated from the seed of D. odorata. Coumarins are secondary metabolites of higher plants, few microorganisms (bacteria and fungi), and sponges [45]. The function of this type of end product of secondary metabolism is related to defense mechanisms against herbivores and attacks by microorganisms. These compounds are biosynthesized from phenylalanine via the shikimic acid [46]. Natural coumarins are generally unsaturated lactones and comprise another class of compounds C6C3. Almost all the natural coumarins have an oxygenated substituent at position 7 [47], either free as in hydroxylated umbelliferone, or combined (methyl, sugars, etc.) in other derivatives. Structurally, they are considered derivatives of the ortho-hydroxy-cinnamic acid.
There are different classifications for the coumarin derivatives. Generally, they can be chemically classified according to the most common cores: simple coumarins, complex coumarins, and various coumarins. More complex coumarins are generally fused with other heterocycles [3]. Therefore, they can be classified as: simple coumarins, furocoumarins, dihydrofurocoumarins, pyranocoumarins (linear and angular), phenylcoumarins, and biscoumarins [1]. As said before, hundreds of coumarins have been identified in natural sources, especially plants [48, 49]. Major coumarin constituents isolated from plants include: simple hydroxycoumarins, furocoumarins and isofurocoumarins, pyranocoumarins, biscoumarins, and dihydroisocoumarins.
3. Biosynthesis of coumarins
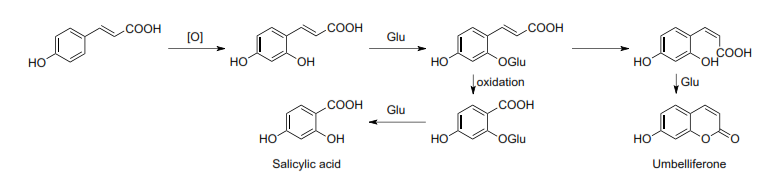
Simple coumarins are biogenetically derived from shikimic acid, via cinnamic acid. The specificity of the process is the C-2 hydroxylation, producing a break (β-oxidation) of the side chain (i.e. Salix spp.), or chain isomerization and subsequent lactonization, generating the umbelliferone. Figure 4 explains the entire process [46, 53].
Pyrano and furocoumarins (Figure 2) are also biogenetically derived from shikimic acid. These coumarins could be divided in two groups—lineal and angular—depending on the position where the isopentenyl pyrophosphate is condensed to further cyclize and form the heterocycle. The biosynthesis of these complex coumarins could also be the result of the cyclization of a simple coumarin previously prenylated [53].
Among the coumarins classified as “various” is the dicoumarol, which is formed by bacterial fermentation of Yellow Sweet Clover, and was isolated for the first time from decomposed leaves of Melilotus albus (Fabaceae/Leguminosae).
An approximation for the dicumarol biogenesis is the hydroxylation of the 4-position of the coumarin, that then captures a molecule of formaldehyde and is condensed with another molecule of 4-hydroxycoumarin, and finally enolize the keto group forming the dicumarol [46].
4. Coumarins in medicinal plants
A large number of valuable species used commonly as medicinal plants, aromatic plants, and edible plants for human and animal feeding belongs to coumarin-rich plant families. Among them are species with well-documented biological activity, in which coumarins are part of the active principles. Table 1 shows a selection of plants of these families (first listed seven families with number of occurrence > 100) and some other families with species of particular pharmacological interest on chronic diseases. Coumarins presenting great pharmacological interest have been isolated in different geographical regions from other botanical families. Also shown are the coumarin compounds having species and their yield (if available).
Most of these plants are well known by people and scientists as part of herbal medicine repertories in Europe, Asia, or the Americas [55-58]. From the list, several coumarin-containing species or genera have also ethnomedical records in Cuba and the Caribbean Basin [59, 60]. Among of plant included are species with a great historical record of ethnomedicinal uses, and are present in traditional medicine systems: Ayurveda Medicine, Traditional Chinese Medicine and Unani Medicine, or in other recent cultures. Also, renowned species used on conventional therapeutics and modern herbal medicine are included, ie. Aesculus hippocastanum (Horse-chestnut), Passiflora incarnata (Passion Flower), Lawsonia inermis (Henna), Hypericum perforatum (Saint John Wort), Tilia cordata (Lime Tree) and Uncaria tomentosa (Cat’s Claw).
Coumarins are also present in several species belonging to different botanical families, which are widespread in the northeastern region of Brazil [61]. Some of them are reported in folk medicine as traditional remedies drugs for the treatment of respiratory diseases [55]. Many pharmacological activities have been ascribed to coumarins such as anticlotting, hypotensive, antimicrobial, anti-inflammatory, and antitumor activities [61].
Recent studies and review manuscripts regarding the coumarin scaffold describe the huge variety of biological activities that may be present in the natural coumarins [8, 18, 62-64]. Venugopala et al. (2013) presented several coumarins displaying activities such as anti-inflammatory, anticoagulant, antibacterial, antifungal, antiviral, anticancer, anti-hypertensive, antitubercular, anticonvulsant, anti-adipogenic, Cytochrome P450 inhibiting, anti-hyperglycemic, antioxidant, and neuroprotective. Several recent reviews summarize and highlight advances in the application of coumarins, especially concerning their antioxidant and anticancer properties [62-70].From Calophyllum spp., it is remarkable the antiviral activity of calanolides and other related pyranocoumarins on Epstein-Barr virus and HIV [56]. As active compound of molluscicidal effects on Biomphalaria glabrata of C. brasiliense extracts were determined (-) mammea A/BB, also found in C. Verticillatum [71].
It is the great structural diversity of coumarinic compounds that allows for their several applications, and also allows for the high interest of these derivatives as phytochemicals. The pharmacological and biochemical properties and therapeutic applications of simple coumarins depend upon the pattern of substitution [68].
5. Natural coumarins, non-nutrients presented in the food
Phytochemicals are defined as bioactive non-nutrient plant compounds presented in fruits, vegetables, grains, and other food plants that have been linked to reducing the risk of major chronic diseases. It is estimated that > 5,000 individual phytochemicals have been identified in fruits, vegetables, and grains, but a large percentage still remain unknown and need to be identified before we can fully understand the health benefits of phytochemicals in whole foods [101].
Phenolics are compounds possessing one or more aromatic rings with one or more hydroxy groups, and generally are categorized as phenolic acids, flavonoids, stilbenes, coumarins, and tannins [102]. Phenolics are the products of secondary metabolism in plants, providing essential functions in the reproduction and the growth of the plants, acting as defense mechanisms against pathogens, parasites, and predators, as well as contributing to the color of plants [103]. In addition to their roles in plants, phenolic compounds in our diet may provide health benefits associated with reduced risk of chronic diseases. Among the 11 common fruits consumed in the United States, cranberry has the highest total phenolic content, followed by apple, red grape, strawberry, pineapple, banana, peach, lemon, orange, pear, and grapefruit [104]. Some of these fruits as important antioxidant and antiproliferative activities [104]. Among the 10 common vegetables consumed in the United States, broccoli possesses the highest total phenolic content, followed by spinach, yellow onion, red pepper, carrot, cabbage, potato, lettuce, celery, and cucumber [105]. Some of these vegetables proved to display interesting antioxidant and antiproliferative activities [105]. It is estimated that flavonoids account for approximately two thirds of the phenolics in our diet and the remaining one third are from phenolic acids [106].
6.Conclusion
Coumarins have been increasingly attracting special interest as phytochemicals due to their underlying outstanding contributions in the prevention and treatment of diseases. Coumarins represent a diverse class of phytochemicals that are ubiquitous in the human diet. Some of the medicinal usages of extracts of plants containing coumarins have been proven in experimental models, which suggested that the extracts possess various pharmacological actions. Several related researches and developments make coumarins an extremely attractive scaffold. The role of coumarins as important phytochemicals and their interesting applications were presented and discussed in this book chapter. The origin, natural sources, biosynthesis, and applications were also described.
You may like
Related articles And Qustion
See also
Lastest Price from Coumarin manufacturers

US $0.00/KG2025-09-03
- CAS:
- 91-64-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $5.00-0.50/KG2025-05-07
- CAS:
- 91-64-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS