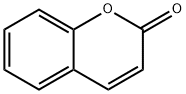
Coumarin Metabolism, Toxicity and Carcinogenicity
Coumarin is a natural product which exhibits marked species dierences in both metabolism and toxicity. The majority of tests for mutagenic and genotoxic potential suggest that coumarin is not a genotoxic agent. The target organs for toxicity and carcinogenicity in the rat and mouse are primarily the liver and lung. Moreover, the dose±response relationships for coumarin-induced toxicity and carcinogenicity are non-linear, with tumour formation only being observed at high doses which are associated with hepatic and pulmonary toxicity. Other species, including the Syrian hamster, are seemingly resistant to coumarin-induced toxicity.
Uses
Coumarin is used as a ®xative and enhancing agent in perfumes and is added to toilet soap and detergents, toothpaste, tobacco products and some alcoholic beverages (Cohen, 1979; Opdyke, 1974).Large quantities are used in rubber and plastic materials and in paints and sprays to neutralize unpleasant odours (Fentem and Fry, 1993).
Because of certain of its biochemical properties, coumarin has been proposed for use in clinical medicine. For example, the ability of coumarin to activate macrophages underlies its use for the treatment of high protein oedema and its immunomodulatory properties used in the treatment of brucellosis (Egan et al., 1990). Coumarin is currently undergoing clinical trials for the treatment of lymphoedema following breast cancer treatment and in the treatment of lung and kidney carcinoma, having been used both in isolation (Thornes et al., 1989) and in combination with cimetidine, as an antineoplastic treatment (Cox et al., 1989; Dexeus et al., 1990; Marshall et al., 1987a, 1989). As a consequence, thousands of individuals have been exposed to therapeutic doses of coumarin ranging from 8 to 7000 mg/day for periods ranging from 2 weeks to over 2 years.
The use of coumarin as a food ¯avour was discontinued as a result of the ®nding of hepatotoxic eects in rats and dogs fed coumarin in the diet (Hazleton et al., 1956). Coumarin was banned in the USA in 1954 based on reports of hepatotoxicity in rats, prior to the existence of any carcinogenicity and mutagenicity data and was recommended for withdrawal from use in the UK in 1965 (Cohen, 1979; Opdyke, 1974).
Occurrence and human exposure
Coumarin is a naturally occurring compound, being present in a wide variety of plants, microorganisms and in some animal species (see Feuer, 1974; Soine, 1964). Coumarin was ®rst isolated from tonka beans, and is found at high levels in some essential oils, particularly cinnamon bark oil (7000 ppm), cassia leaf oil (up to 87,300 ppm) and lavender oil. Coumarin is also found in fruits (e.g. bilberry, cloudberry), green tea and other foods, such as chicory (TNO, 1996). Many coumarin derivatives have also been identi®ed in plants, being present both in the free state and as glucosides.
Coumarin is listed as an ``active principle'' by the Council of Europe and the maximum permitted concentrations in foodstus are given in Annex II of European Directive (88/388/EEC). The general limit for coumarin in food and non-alcoholic beverages is 2 mg/kg; however, in alcoholic beverages and certain caramel confectionery, the permitted limit is 10 mg/kg and in chewing gum it is 50 mg/ kg
Based on intake data for food, beverages, caramel confectionery, chewing gum and alcoholic beverages, a theoretical maximum daily intake of coumarin can be calculated to be 4.085 mg/day or 0.07 mg/kg/day for a 60-kg consumer. This theoretical value contains a large contribution from the consumption of general foods and is unrealistically too high. It has to be corrected, keeping in mind real use of coumarin-containing ingredients. The main potential sources of coumarin in the diet are the coumarin content of cinnamon, the use of Asperula odorata in ``Maiwein'' and of Hierochloe odorata in special vodka.
It would be unreasonable to assume that the solid food dietary budget for a typical individual would be made up of 1500 g cinnamon-¯avoured foodstus. This is particularly so since no ¯avours are added to most food items. It would be more reasonable to assume that over a long period of time less than 5% of solid food would be ¯avoured with cinnamon or other ingredients capable of imparting the 2 mg/kg maximum concentration of coumarin. This would reduce maximum daily intake to 1.235 mg/day or 0.02 mg/kg/day for a 60-kg consumer (F. Grundschober, personal communication).
Absorption, distribution, metabolism and excretion of coumarin
There is an extensive literature on the disposition and metabolism of coumarin in a range of animal species and humans. Metabolic studies have been performed both in vivo and in vitro.
Following oral administration, coumarin is rapidly absorbed from the gastrointestinal tract and is distributed throughout the body (Cohen, 1979; Fentem and Fry, 1993; Pelkonen et al., 1997). The compound appears to be extensively metabolized in all species with little unchanged coumarin being excreted (see below). No evidence of signi®cant tissue accumulation of coumarin and/or coumarin metabolites was obtained after oral administration to rats and rabbits (Kaighen and Williams, 1961) or intraperitoneal (ip) administration to rats (van Sumere and Teuchy, 1971)
Some representative data for the routes of excretion of coumarin metabolites are shown in Table 2. There are important quantitative dierences between species in the routes of elimination of coumarin metabolites. The majority of studies have demonstrated a relatively large amount of biliary excretion in the rat with an appreciable proportion of the dose being excreted in the faeces (Table 2). Indeed, after a 50 mg/kg oral or ip dose of coumarin to rats some 50% of the dose was excreted in the bile as unknown metabolites within 24 hours (Williams et al., 1965). The urine appears to be the major route of coumarin metabolite excretion in species such as the Syrian hamster, rabbit and baboon (Table 2). The rapid excretion of coumarin, primarily as 7-hydroxycoumarin (7-HC), in the urine of human subjects given coumarin suggests that there is little or no biliary excretion of coumarin metabolites in humans (Shilling et al., 1969).
Genotoxicity studies
A number of studies have examined the mutagenic and genotoxic potential of coumarin. Overall, the data suggest that coumarin is not a genotoxic agent. No evidence for coumarin-induced genotoxicity has been observed in in vivo studies (Drosophila melanogaster and mouse micronucleus tests). The ®ndings from these in vivo studies are supported by data from some, but not all, of the in vitro studies. Of signi®cance is the lack of eect of coumarin on unscheduled DNA synthesis (UDS) in human liver slices. In addition, neither o-HPAA nor 7-HC (i.e. the major metabolites of coumarin in the rat and human, respectively) induced UDS in rat hepatocytes or produced any evidence of mutagenicity in ®ve Salmonella typhimurium strains. These various in vivo and in vitro studies are discussed in detail below.
In in vivo studies coumarin did not induce sexlinked recessive lethal mutations in germ cells of male D. melanogaster exposed as adults either by feeding or by injection (NTP, 1993a; Yoon et al., 1985), or as larvae by feeding (NTP, 1993a; Valencia et al., 1989). The administration of 65 and 130 mg/kg/day coumarin by gavage for 7 days did not induce micronuclei in the bone marrow of male and female ICR mice (Morris and Ward, 1992). Moreover, in male, but not in female, ICR mice coumarin treatment was found to decrease micronuclei formation by benzo[a]pyrene. Coumarin did not induce micronuclei in peripheral blood erythrocytes of male and female B6C3F1 mice given 75±300 mg/ kg coumarin by gavage 5 days per week for 13 weeks (NTP, 1993a).
A number of in vitro genotoxicity studies have been conducted with coumarin. Recently the eect of coumarin on unscheduled DNA synthesis (UDS) in cultured precision-cut human liver slices has been evaluated. Previous work has demonstrated that like hepatocytes, cultured liver slices may also be employed to screen xenobiotics for eects on UDS (Lake et al., 1996). No evidence for coumarininduced UDS was obtained in experiments with cultured liver slices from four subjects employing coumarin concentrations of up to 5 mM (Beamand et al., 1998). Some cytotoxicity was observed at high coumarin concentrations and the functional viability of the human liver slice preparations was con®rmed by parallel studies with known genotoxins. Coumarin has also been reported not to induce UDS in a rat tracheal epithelium culture system (Ide et al., 1981).
Toxicity and carcinogenicity studies
A number of studies have examined the acute, chronic and carcinogenic eects of coumarin in the rat and mouse. The long-term studies have been performed at maximally tolerated doses, with coumarin intakes over 4500 times the estimated human exposure from the diet and from fragrance use in cosmetic products. Moreover, these studies demonstrate that threshold doses exist for coumarininduced toxicity and carcinogenicity, below which such eects are not observed.
Conclusions
By examination of the available literature a number of conclusions may be drawn, which include:
(i) Coumarin exhibits marked species dierences in both metabolism and toxicity.
(ii) The majority of the available data suggest that coumarin is not a genotoxic agent.
(iii) Hepatotoxicity in the rat, mouse and dog, but not in baboons or humans, is probably associated with the predominance of the 7-hydroxylation detoxi®cation pathway in the latter species. However, 7-hydroxylation status is not the only determinant of susceptibility of a species to coumarin-induced toxicity.
(iv) Evidence of carcinogenicity to rat and mouse liver and to mouse lung is probably attributable to non-genotoxic mechanisms which only operate when there is target organ damage. For both tissues threshold doses have been established below which no toxicity or increased tumours are observed.
(v) Comparison of the NOELs for coumarininduced toxicity and carcinogenicity with estimated human exposure from the diet and cosmetic products provides acceptable safety margins, such that coumarin exposure from these sources should pose no health risk to humans.
Related articles And Qustion
See also
Lastest Price from Coumarin manufacturers

US $5.00-0.50/KG2025-05-07
- CAS:
- 91-64-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/KG2025-04-21
- CAS:
- 91-64-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month