Bupivacaine hydrochloride--Metabolism, Excretion, Pediatric use and recommended dose
Classification. Amide.
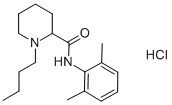
Chemical formula. 1-Butyl-2′,6′-pipecoloxylidide hydrochloride; structurally related to mepivacaine except for a butyl group replacing a methyl group.
Prepared by. A.F. Ekenstam, 1957.
Articaine box showing “1.7 mL each.” (Courtesy of Septodont, Inc., Lancaster, PA.)
FDA approved. October 1972.
Potency. Four times that of lidocaine, mepivacaine, and prilocaine. Toxicity. Less than four times that of lidocaine and mepivacaine. Metabolism. Metabolized in the liver by amidases.
Excretion. Via the kidney; 16% unchanged bupivacaine has been recovered from human urine.
Vasodilating properties. Relatively significant: greater than those of lidocaine, prilocaine, and mepivacaine, yet considerably less than those of procaine.
pKa. 8.1.
pH of plain solution. 4.5 to 6.0.
pH of vasoconstrictor-containing solution. 3.0 to 4.5.
Onset of action. Slower onset than other commonly used local anesthetics (e.g., 6 to 10 minutes).
Effective dental concentration. 0.5%.
Anesthetic half-life. 2.7 hours.
Topical anesthetic action. Not in clinically acceptable concentrations.
Pregnancy classification. C.
Nursing mothers. The FDA states that it is not known whether local anesthetic drugs are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when local anesthetics are administered to a nursing woman. Health Canada (equivalent to the US FDA) states that bupivacaine is excreted in the breast milk, but in such small quantities that there is generally no risk of affecting the infant at therapeutic doses.
Bupivacaine, 0.5%, with epinephrine 1:200,000. (A) Marcaine. (B) Vivacaine. ([A] Courtesy, Cook-Waite, Carestream Dental LLC, Atlanta, Georgia, United States, [B] Courtesy of Septodont, Inc., Lancaster, PA.)
Pediatric use. Until further experience is gained in children younger than 12 years, administration of bupivacaine in this age group is not recommended, and the Canadian drug package insert states: Until further experience is gained, bupivacaine is not recommended for children younger than two years of age.
Maximum recommended dose. The FDA MRD of bupivacaine is 90 mg. There is no recommended dose for bupivacaine based on body weight in the United States. In Canada, the MRD is 2.0 mg/kg to a maximum of 200 mg. For children older than 2 years, in Canada the maximum dose of bupivacaine is based on 2.0 mg/kg (0.9 mg/lb).
Comments. Bupivacaine has been available in cartridges since February 1982 in Canada and since July 1983 in the United States. Bupivacaine is available as a 0.5% solution with epinephrine 1:200,000; there are two primary indications for its use in dentistry:
1. lengthy dental procedures for which pulpal (deep) anesthesia in excess of 90 minutes is necessary (e.g., full mouth reconstruction, implant surgery, extensive periodontal procedures)
2. management of postoperative pain (e.g., endodontic, periodontal, exodontia)
The patient’s requirement for postoperative opioid analgesics is considerably lessened when bupivacaine is administered for pain control.78 For postoperative pain control after a short surgical procedure (<30 minutes), bupivacaine may be administered at the start of the procedure following administration of the local anesthetic chosen for periprocedural pain management (e.g., articaine, lidocaine, mepivacaine, prilocaine). However, for postoperative pain control after lengthy surgical procedures, it is reasonable to administer bupivacaine at the conclusion of the procedure, just before the patient’s release from the dental operating chair.
A protocol for management of postsurgical pain has been developed that is clinically quite effective. It combines the administration of preoperative and postoperative nonsteroidal antiinflammatory drugs with injectable local anesthetics for procedural and postoperative pain management.
The onset of anesthesia with bupivacaine is commonly delayed for 6 to 10 minutes, which is understandable in view of its pKa of 8.1. If bupivacaine is being used for pain control during a lengthy dental procedure, it may be advisable to initiate procedural pain control with a more rapidly acting amide (e.g., articaine, mepivacaine, lidocaine, prilocaine), allowing the procedure to commence more promptly. This should be followed with the administration of bupivacaine for increased duration of pain control.
Bupivacaine is not recommended in younger patients (younger than 12 years in the United States; younger than 2 years in Canada) or in those for whom the risk of postoperative soft tissue injury produced by self-mutilation is increased, such as physically and mentally disabled persons. Bupivacaine is rarely indicated in children because pediatric dental procedures are usually of short duration and the increased risk of self-inflicted soft tissue injury (e.g. chewing of the lip or tongue) after the child has left the dental office.
You may like
Related articles And Qustion
Lastest Price from Bupivacaine hydrochloride manufacturers

US $480.00-230.00/kg2025-04-21
- CAS:
- 14252-80-3
- Min. Order:
- 1kg
- Purity:
- 99%pure
- Supply Ability:
- 1000kg

US $480.00-230.00/kg2025-04-21
- CAS:
- 14252-80-3
- Min. Order:
- 1kg
- Purity:
- 99%pure
- Supply Ability:
- 1000kg