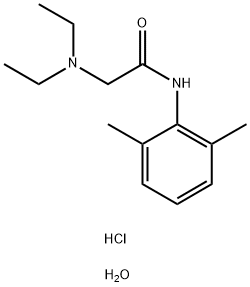
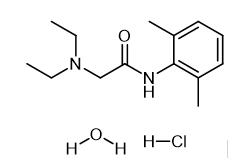
Lidocaine hydrochloride: A local anesthetic
Lidocaine hydrochloride is a local anesthetic (numbing medication) that is used to numb an area of your body to help reduce pain or discomfort caused by invasive medical procedures such as surgery, needle punctures, or insertion of a catheter or breathing tube. Lidocaine hydrochloride injection is sometimes used to treat irregular heart rhythms that may signal a possible heart attack. Lidocaine hydrochloride injection is also given in an epidural (spinal block) to reduce the discomfort of contractions during labor. Lidocaine injection may also be used for purposes not listed in this medication guide.

Synthesis of Lidocaine hydrochloride
Distillation apparatus is provided with three sequentially in the bottle by adding sodium methoxide 15.12g (0.28mol), intermediate 2,6-dimethyl aniline 24.24g (0.2mol) and N, N-diethyl amino methyl acetate 31.9g (0.22mol), heating to 95 °C, side reaction-side distillation to remove reaction generated methanol, the methanol is distilled until the free, to continue the reaction 30 min, cooling to room temperature, dissolved in dichloroethane, washing 2 times, static hierarchical, organic layer is lidocaine dichloroethane solution of alkali. The lidocaine in dichloroethane solution of alkali, adding hydrochloric acid 2.56g, then with hydrogen chloride for adjusting PH 3.5, reflux adding activated carbon to 20 min, filtering, concentrating the filtrate, the cooling crystallization, drying to obtain the lidocaine hydrochloride 48.28g, HPLC purity is 99.52%, the yield is 88.72%, overall yield is 84.04%.[1]
Indications
Local anesthetics are classified into 2 primary categories: esters and amides. Esters (eg, cocaine, procaine, chloroprocaine, tetracaine) are metabolized by the enzyme pseudocholinesterase. In contrast, the liver metabolizes amides (eg, Lidocaine hydrochloride, bupivacaine, prilocaine, and ropivacaine). Formerly referred to as lignocaine, Lidocaine hydrochloride is a tertiary amine anesthetic agent derived from xylidine and was first synthesized between 1943 and 1946 by Nils Löfgren and Bengt Lundquist. This medication rapidly became used worldwide, given its superior safety profile compared to older local anesthetic agents. Lidocaine hydrochloride is commonly used for local anesthesia and is often combined with epinephrine (which extends Lidocaine hydrochloride's duration of action by opposing the local vasodilatory effects of Lidocaine hydrochloride). Lidocaine hydrochloride can also be administered intravenously during tracheal intubation, obtunding the hypertensive response to laryngoscopy and potentially reducing the incidence of myalgia and hyperkalemia after succinylcholine is given. According to the Vaughan-Williams classification, Lidocaine hydrochloride is a class Ib antiarrhythmic agent, and its use is indicated in the management of acute ventricular tachyarrythmias. Lidocaine hydrochloride is also FDA-approved as a treatment for ventricular dysrhythmias after cardiac surgery.[2]
Lidocaine hydrochloride may also be used as an adjuvant analgesic for patients with acute and chronic pain. According to the American Society of Regional Anesthesia and Pain Medicine (ASRA), perioperative Lidocaine hydrochloride infusion may be a valuable adjunct in Enhanced Recovery After Surgery (ERAS) protocols for improved pain management. The American College of Cardiology/American Heart Association (ACC/AHA) recommends considering Lidocaine hydrochloride as a component of Advanced Cardiovascular Life Support (ACLS) interventions for patients experiencing cardiac arrest due to polymorphic ventricular tachycardia or ventricular fibrillation. According to the American Heart Association (AHA), American College of Cardiology (ACC), and Heart Rhythm Society (HRS), amiodarone or Lidocaine hydrochloride may be considered for patients with ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT) that does not respond to defibrillation. These medications can be beneficial for patients with a witnessed cardiac arrest, as they may be administered relatively quickly.
The Difficult Airway Society guidelines for awake tracheal intubation (ATI) suggest that topical Lidocaine hydrochloride offers up to 40 minutes of analgesia, with variability based on concentration and administration. The return of laryngeal reflexes may be delayed. Due to Lidocaine hydrochloride's terminal elimination half-life of up to 2 hours, patients should remain nil per os (NPO) for at least 2 hours post-application. Perioperative Lidocaine hydrochloride infusion may be beneficial for bariatric patients, who are often more sensitive to the respiratory depressant effects of opioids. In patients undergoing bariatric surgery, Lidocaine hydrochloride infusion has been shown to reduce 24-hour opioid consumption by 10 mg morphine equivalents compared to placebo, which is associated with improved recovery. Lidocaine hydrochloride is a potential treatment for chronic pain. Evidence for Lidocaine hydrochloride's efficacy is less robust for patients with complex regional pain syndrome (CRPS) and cancer. However, Lidocaine hydrochloride demonstrates significant efficacy as an adjunctive therapy for chronic post-surgical pain. Continued research is needed to understand better the mechanisms underlying Lidocaine hydrochloride's effects on pain pathways. Lidocaine hydrochloride and steroids can effectively alleviate chronic cervical radiculopathy symptoms using an ultrasound-guided selective nerve root block technique. Epidural analgesia with fentanyl and Lidocaine hydrochloride is equivalent to intrathecal fentanyl for pain relief during early labor, with similar efficacy, duration, and patient satisfaction.
Mechanism of Action
Similar to other local anesthetics, Lidocaine hydrochloride acts at sodium ion channels on the internal surface of nerve cell membranes. The uncharged form of Lidocaine hydrochloride diffuses through neural sheaths into the axoplasm before ionizing by combining with hydrogen ions. The resulting cation binds reversibly to sodium channels from the inside, locking them in the open state and preventing nerve depolarization. Lidocaine hydrochloride is a weak base with a dissociation constant (pKa) of 7.7. Approximately 25% of Lidocaine hydrochloride molecules are neutrally charged at a physiological pH of 7.4 and can translocate inside the nerve cells, meaning Lidocaine hydrochloride has a more rapid onset of action than other local anesthetics with higher pKa values.
Lidocaine hydrochloride's efficacy is reduced at inflammation sites; this may be due to acidosis reducing the proportion of neutral Lidocaine hydrochloride molecules, faster reductions in local Lidocaine hydrochloride concentration due to increased blood flow, or increased production of inflammatory mediators like peroxynitrite, which act directly on sodium channels. Lidocaine hydrochloride's function as an NMDA antagonist offers potential benefits for patients with difficult-to-control pain, such as mixed nociceptive-neuropathic pain and central sensitization. In cardiac myocytes, Lidocaine hydrochloride slows the rise of the cardiac action potential during phase 0, increasing the effective threshold potential. Increased blood levels of Lidocaine hydrochloride can affect cardiac output, total peripheral resistance, and mean arterial pressure, likely due to its direct depressant effects on the cardiovascular system.
Toxicity
Signs and symptoms of mild toxicity become apparent at plasma levels greater than 5 μg/mL, beginning with slurred speech, tinnitus, circumoral paresthesia, and lightheadedness. Above 10 μg/mL, the patient may experience seizures or loss of consciousness. The myocardium and central nervous system are further depressed at 15 μg/mL, progressing to cardiac arrhythmias, respiratory arrest, and cardiac arrest above 20 μg/mL. Animal studies suggest that the dose of Lidocaine hydrochloride required to cause cardiovascular collapse is 7.1 ± 1.1 times higher than the dose needed to induce central nervous system effects. This so-called "(cardiovascular collapse/CNS (CC/CNS) ratio" is significantly higher than the ratio for other local anesthetic agents; bupivacaine has a CC/CNS ratio of around 2.0. In the event of toxic dosing in the conscious patient, Lidocaine hydrochloride may be less likely than other local anesthetics to progress rapidly from neurological effects to complete cardiovascular collapse. By contrast, neurological signs and symptoms can often be masked if the patient is under the concurrent effects of sedation or general anesthesia, meaning that cardiovascular instability or arrhythmias may be the first manifestations.[3]
References
[1] ZHEJIANG ESIGMA BIOTECHNOLOGY - CN105294477, 2016, A
[2] Beecham GB, Nessel TA, Goyal A. Lidocaine. [Updated 2024 Aug 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-.
[3] Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012 Summer;59(2):90-101; quiz 102-3.
You may like
Related articles And Qustion
See also
Lastest Price from Lidocaine hydrochloride manufacturers

US $1.00/kg2025-06-10
- CAS:
- 6108-05-0
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 2000kgs

US $5.00-0.50/KG2025-06-05
- CAS:
- 6108-05-0
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS