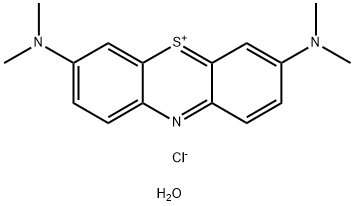
Applications of Methylene Blue
Properties of methylene blue
The chemical formula of methylene blue is C16H18ClN3S, and the relative molecular weight is 319.85. Also known as alkaline Blue 9. Dye index CI basic blue 9 (52015). Dark green crystal or powder, with metallic luster, containing three, four or five crystalline water molecules, stable in air. Tasteless. Soluble in water and ethanol, it is sky blue. It is also soluble in butanol and pentanol.

Figure 1 methylene blue powder
Application of methylene blue in dyeing
It can be used in the manufacture of ink and lakes and the dyeing of biological and bacterial tissues. It can be used for dyeing cotton, hemp, silk fabrics, paper and bamboo and wood. It can also be mixed with crystal violet and yellow dextrin in the ratio of 78:13:9 to form alkaline magenta blue[2].
Role of methylene blue in medical treatment
Because methylene blue has reducibility, its injection is used to treat methemoglobinemia. It is also used to rescue nitrobenzene, nitrite and cyanide poisoning. Mild carbon monoxide poisoning can be detoxified by intravenous injection of methylene blue. It is clinically used to treat sulfonamide allergy[3]. Because of its bactericidal effect, oral methylene blue or washing with its solution can treat cystitis and urethritis. In addition, methylene blue will be discharged from the urine within 30 minutes (injection) to a few hours (oral), resulting in the temporary blue of the urine, so it is also used for the determination of renal function. In the cultivation of ornamental fish, 0.1-0.2ppm methylene blue solution is also used for disinfection or treatment of diseases such as white spot disease.
Application of methylene blue in analysis and identification
In chemical experiments, analytical pure methylene blue can be used as an adsorption indicator in chemical reagents, as well as for the precipitation of perchlorate and rhenium, and the catalytic spectrophotometric determination of selenium and molybdenum. At the same time, methylene blue is also oxidizing. It can oxidize some substances with strong reducibility and be reduced to colorless reduced methylene blue (some people call it methylene white). After being reduced, the reduced methylene blue has certain reducibility, and can be oxidized by some oxidizing substances, such as oxygen in the air, to produce oxidized blue methylene blue. Therefore, methylene blue can be used for oxidation-reduction titration and demonstration of oxidation-reduction oscillating reaction. The most typical is the blue bottle experiment.
Efficacy of methylene blue
Methylene blue is not an officially restricted drug. It is mainly used to control water mildew and small melon insects. It can replace malachite green. Methylene blue dissolved in water does little harm to fish. In addition, the drug has made achievements in many fields such as industry, medicine and skin care. Even the drug will be applied to more difficult cancer diseases. Methylene blue is more effective in the treatment of non advanced fish diseases. There are snails, snails, snails, snails, snails, snails, snails, snails, etc. Methylene blue is an oxidant, which can oxidize nitrite into nitrate, inhibit and kill bacteria. However, it also has a certain impact on nitrifying bacteria, so some measures should be taken after use.
Harm of methylene blue
Methylene blue solution with high concentration (5 ~ 10mg / kg; 1% solution 25 ~ 50ml) oxidizes hemoglobin to produce methemoglobin. The reason is that when a large amount of this product enters the body, the production of reduced coenzyme I dehydrogenase (NADPH) is reduced, which can not change all this product into reduced methylene blue. There is a large amount of oxidized methylene blue, and hemoglobin is oxidized to methemoglobin. When the intravenous injection of methylene blue solution is too large (500mg), it can cause adverse reactions such as nausea, abdominal pain, precordial pain, dizziness, headache, sweating and unconsciousness. In addition, the substance may be harmful to the environment, so special attention should be paid to the water body
Detection of methylene blue
Take about 10mg of this product, add 50ml of water to dissolve it, and it will appear dark blue; Separate 10ml of solution, add 1ml of dilute sulfuric acid and 0.1g of zinc powder, the blue will disappear, filter, put the filtrate in the air or add 1 drop of hydrogen peroxide test solution to reappear the blue; Take another 10ml of solution and add several drops of potassium iodide test solution to form dark blue villous precipitation. The upper layer is light blue, and the upper layer is light blue; Then take 10ml of the solution and add 0.1mol/l iodine solution drops, which is dark brown; Add 0.1mol/l sodium thiosulfate solution to regain blue.
Preparation of methylene blue
Methylene blue can be nitrosated from n, N-dimethylaniline and reduced to p-aminodimethylaniline, then oxidized, vulcanized and condensed with sodium dichromate and sodium thiosulfate, and then salted, salted out, filtered and dried with zinc chloride. In the industrial preparation of methylene blue, first add 100kg pure water to 10kg of industrial methylene blue (alkaline lake blue BB), stir and steam to 80 ~ 90 ℃ to dissolve it. Then slowly add 7.5kg of 20% sodium carbonate hot solution for 30min, continue stirring for 10min, after standing for half an hour, heat the solution, the temperature shall not exceed 90 ℃, filter while it is hot, add 3kg 1:1 hydrochloric acid into the clear filtrate, stir evenly, cool and crystallize, after the crystallization is complete, centrifuge and dry it at 40 ~ 50 ℃.
Reference
1 Jack Clifton I I, Leikin J B. Methylene blue[J]. American journal of therapeutics, 2003, 10(4): 289-291.
2 Ginimuge P R, Jyothi S D. Methylene blue: revisited[J]. Journal of anaesthesiology, clinical pharmacology, 2010, 26(4): 517.
3 Mayer B, Brunner F, Schmidt K. Novel actions of methylene blue[J]. European heart journal, 1993, 14: 22-26.
);You may like
See also
Lastest Price from Methylene Blue trihydrate manufacturers

US $6.00/KG2024-04-19
- CAS:
- 7220-79-3
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/MONTH
US $1.10/g2024-04-17
- CAS:
- 7220-79-3
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min