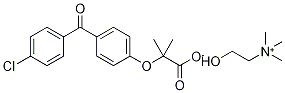
Choline fenofibrate, a salt formulation of fenofibric acid, is a lipid
regulating agent available as delayed release capsules for oral administration. It is indicated in combination with a statin to reduce TG and
increase high-density lipoprotein cholesterol (HDL-C) in patients with
mixed dyslipidemia and coronary heart disease (CHD) or a CHD-risk
equivalent who are optimal for statin therapy to achieve their lowdensity lipoprotein cholesterol (LDL-C) goal. In addition, choline
fenofibrate is indicated as monotherapy in patients with severe
hypertriglyceridemia to reduce TG, and in patients with primary
hyperlipidemia or mixed dyslipidemia to reduce elevated LDL-C, total
cholesterol, TG, apolipoprotein B, and to increase HDL-C. Fenofibric acid
is also the active metabolite of fenofibrate (Tricor), which has been
previously marketed for the treatment of hypercholesterolemia and
hypertriglyceridemia.
The primary mode of action of fenofibric acid
is through the activation of the nuclear transcription factor PPARa,
predominantly expressed in tissues that metabolize fatty acids, such as
the liver, kidney, heart, and muscle.
On activation by binding of the fibrate, PPARa binds as heterodimers with retinoid X receptor (RXR), which subsequently recognizes and binds to specific PPARa-response elements leading to modulation of expression of the target genes. In particular, the activity of lipoprotein lipase is increased and synthesis of apoprotein C-III is decreased, which together enhance the clearance of circulating TG-rich lipoproteins. The resulting fall in TG produces an alteration in the size and composition of LDL from small dense particles to large buoyant particles. These larger particles have a greater affinity for cholesterol receptors and are catabolized rapidly. Choline fenofibriate acid is contraindicated in patients with severe renal impairment.