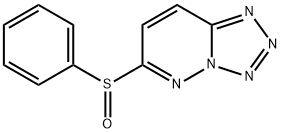
Transcriptional activation by NF-κB is limited by IκBα binding, which masks the NF-κB nuclear localization signal that initiates nuclear translocation. Cytokines trigger a signaling cascade of kinases that target IκBα for degradation, enabling NF-κB to promote gene expression. Because NF-κB regulates the transcription of inflammatory mediators, blocking its signaling activity is regarded as an effective means to treat inflammatory diseases. Ro 106-9920 is a small molecule inhibitor of NF-κB-dependent expression of TNF-α, interleukin-1β, and interleukin-6 (IC50 < 1 μM in human peripheral blood mononuclear cells). It selectively inhibits the ubiquitination of activated IκBα with an IC50 value of 2.3 μM demonstrating little activity against the ubiquitin-activating enzyme E1 (IC50s >100 μM), the ubiquinating-conjugating enzyme E2UBCH7, nonspecific ubiquitination of cellular proteins, and 97 other molecular targets. In two different models of acute inflammation, Ro 106-9920 at 10-100 mg/kg has been shown to dose-dependently inhibit cytokine production in rat.