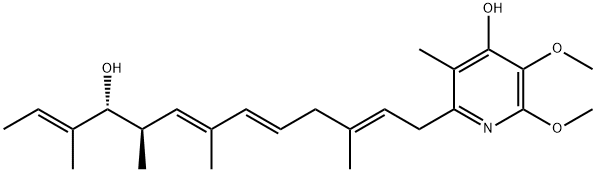
Complex I, also known as NADH:ubiquinone oxidoreductase or NADH dehydrogenase (ubiquinone), catalyzes the transfer of electrons from NADH to ubiquinone (also known as coenzyme Q10) as part of the respiratory chain leading to ATP generation. Piericidin A is an irreversible inhibitor of mitochondrial complex I that strongly associates with ubiquinone binding sites in both mitochondrial and bacterial forms of the enzyme. First identified as an insecticidal metabolite produced by Streptomyces, piericidin A was soon found to bind and inhibit complex I at nanomolar concentrations. The inhibition of complex I by piericidin A in the presence of NADH results in the generation of reactive oxygen species. In plants, pieridicin A inhibits photosystem II, a water-plastoquinone oxidoreductase involved in light-dependent electron transfer. Piericidin A also suppresses the up-regulation of the glucose-regulated protein GRP78 in glucose-deprived, etoposide-resistant HT-29 cells, resulting in cell death (IC50 = 7.7 nM).