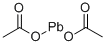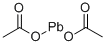Lead
acetate is stable under ordinary conditions of use and storage. Lead acetate is incompatible
with bromates, phenol, chloral hydrate, sulphides, hydrogen peroxide, resorcinol,
salicylic acid, sulphites, vegetable infusions, alkalis, tannin, phosphates, citrates, chlorides,
carbonates, tartrates, and acids. Lead (II) acetate, as well as white lead, has been
used in cosmetics throughout history, though this practice has ceased in Western countries.
It is still used in men’s hair colouring. Lead (II) acetate paper is used to detect the
poisonous gas hydrogen sulphide. The gas reacts with lead (II) acetate on the moistened
test paper to form a grey precipitate of lead (II) sulphide.