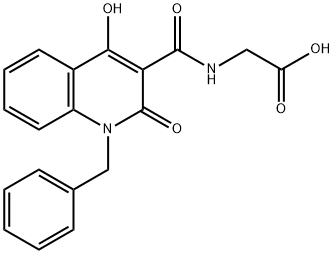
Hypoxia-inducible factor (HIF) is regulated by the hydroxylation of prolyl residues in oxygen-dependent degradation domains in the HIF-1α subunit, which mark it for degradation by the proteasome. HIF prolyl hydroxylation is catalyzed by prolyl hydroxylase domain enzymes (PHD1, 2, and 3), members of the Fe(II) and 2-oxoglutarate (2OG) oxygenase family. They require dioxygen as a cosubstrate, thus acting as the hypoxia-sensing component of the HIF system. The activity of PHD is suppressed by hypoxia, increasing both the abundance and activity of the HIF transcriptional complex. IOX2 potently inhibits PHD2 (IC50 = 21 nM) with over 100-fold selectivity compared to inhibition of JMJD2A, JMJD2C, JMJD2E, JMJD3, or the 2OG oxygenase FIH (IC50s > 100 μM). IOX2 is active in cells, inhibiting HIF-1α hydroxylation in RCC4 cells at 50 μM. See the Structural Genomics Consortium (SGC) website for more information.