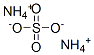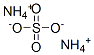Ammonium sulfate, also known as ammonium sulfate, is the earliest production and use of nitrogen fertilizer in domestic and foreign. It is usually used as a standard nitrogen fertilizer, nitrogen content is between 20% to 30%.
In the 1960s, Ammonium sulfate is the main variety of nitrogen fertilizer, but also is a major source to provide crop nutrients sulfur. Firstly ammonia and sulfuric acid was neutralized to obtain, but later increasing proportion of by-product ammonium sulfate, and now basically all domestic-product is from byproduct of other industries, such as coking industry, caprolactam, sulfuric acid tail gas desulfurization, desulfurization of power plant, acrylonitrile and methyl methacrylate, zinc oxide. By-product ammonium sulfate followed the principle of " waste control by waste ", to achieve the comprehensive utilization of waste, to achieve energy saving purposes. Especially with the breakthrough technology of ammonia desulfurization project, by-product ammonium sulfate production of power plant desulfurization will increase substantially in the future.
Pure product of ammonium sulfate is white crystals, heated to 100 ℃ , began to be decomposed into ammonia and ammonium bisulfate, a by-product with a yellowish or gray, small moisture absorption, easy to agglomerate, it is easier to save and easily soluble in water, insoluble ethanol and acetone.
Ammonium sulfate serves as physiological acidic nitrogen fertilizer, is generally more suitable for wheat, corn, rice, cotton, potato, hemp, fruit trees, vegetables and other crops. For soils, the ammonium sulfate is most suitable for neutral soil and alkaline soil, but not suitable for acidic soil. Also used as analytical reagents (precipitating agent, masking agent), in electrochemical analysis, supports electrolyte, microbiological culture media and preparation of ammonium salts.
The above information is edited by the Chemicalbook of Liu Yujie.