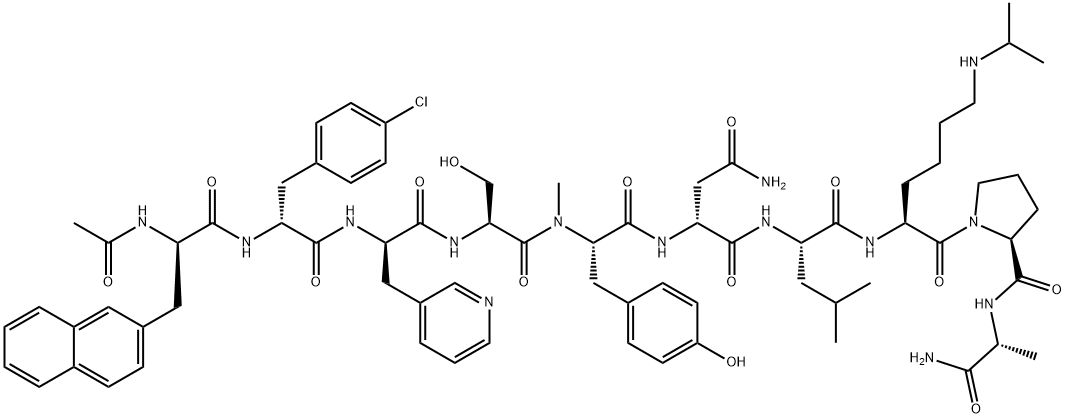
Abarelix is an antagonist of the gonadotropin releasing-hormone (Gn RH) receptor,
and it was launched as an intramuscular injection for the palliative treatment
of advanced symptomatic prostate cancer. Hormonal therapy of prostate
cancer is based on the modulation of testosterone to achieve medical castration
levels. The inhibition of GnRH activity causes the suppression of luteinizing hormone
(LH) and follicle stimulating hormone (FSH) secretion, thereby reducing the
secretion of testosterone by the testes. Abarelix is the first GnRH antagonist to
reach its market. Hormonal therapy with GnRH agonists such as leuprolide, buserelin,
and goserelin has been in use for over two decades. Drugs of this type achieve
the suppression of LH and FSH by a feedback inhibition mechanism, which involves
an initial rise in the LH and FSH levels and, consequently, in the levels of
testosterone. A testosterone surge may induce a clinical tumor flare that worsens cancer-related symptoms. This phenomenon is observed in 4–33% of patients receiving
a GnRH agonist. A GnRH antagonist such as abarelix acts by direct inhibition
of LH and FSH secretion, which avoids the initial surge in serum
testosterone concentrations. Abarelix is a decapeptide, and it is prepared by a
typical coupling cycle for peptide synthesis using Boc-amino acids and a methylbenzhydrylamine
(MBHA) resin. Abarelix has high binding affinity for GnRH
receptor (Kd=0.1 nM). Following intramuscular administration of a 100 mg dose,
abarelix is absorbed slowly with a Cmax of 43.4 ng/mL observed approximately 3
days after the injection and has a half-life of about 13 days. The apparent volume of
distribution is over 4000 L, suggesting extensive distribution. Abarelix has high
protein binding (96–99%), and it is primarily metabolized via hydrolysis of peptide
bonds. Following a dose of 15 μg/kg in humans, approximately 13% of abarelix is
recovered unchanged in the urine, with no detectable metabolites. The renal clearance
of abarelix is 14.4 L/day following a 100 mg dose. Two randomized, open
label, comparative clinical trials involving 348 patients demonstrated the efficacy of
abarelix versus a GnRH agonist (leuprolide) as well as a combination of GnRH
agonist and anti-androgen (leuprolide+bicalutamide). In these trials, both abarelix
and the comparators reduced testosterone to medical castration levels (<50 ng/dL)
by day 29 of therapy in 94–98% of the patients. However, a significant difference
was observed between the two groups for the occurrence of testosterone surge (0%
in the abarelix group versus 82% in the GnRH agonist group) and for the rapidity
of attaining castration levels (72% versus 0% on day 8 in the abarelix and GnRH
agonist groups, respectively). Both groups maintained medical castration levels of
testosterone with similar efficacy between days 29 and 85 of treatment. Abarelix was
generally well tolerated in these trials. Approximately 3% of the patients experienced
an immediate-onset allergic reaction. Other adverse events were similar to
comparator controls and included hot flushes, sleep disturbance, pain, and breast
enlargement. The recommended dosage of abarelix is 100 mg intramuscular injection
on days 1, 15, and 29 of therapy, and every 4 weeks thereafter.