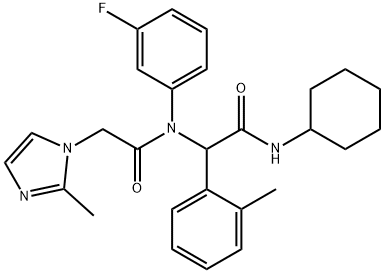
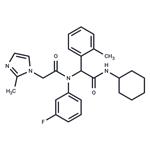
Isocitrate dehydrogenases (IDHs) are nicotinamide adenine dinucleotide (NAD+) and NAD phosphate (NADP+)-dependent enzymes in the tricarboxylic acid cycle that catalyze oxidative decarboxylation of isocitrate producing a-ketoglutarate (2-OG) and carbon dioxide. IDH1 and IDH2 are mutated in >70% of lower grade gliomas. The most common mutations map to arginine residues in the catalytic pockets of IDH1 (R132) or IDH2 (R140 and R172) and impart new gain of function catalytic activity leading to the NADPH-dependent conversion of 2-OG to 2-hydroxyglutarate (2-HG). AGI-5198 is a potent, selective inhibitor of IDH1 R132H and R132C mutants in vitro with IC50 values of 0.07 and 0.16 μM, respectively, but not wild-type IDH1, wild-type IDH2, or IDH2 mutants (IC50s > 100 μM). AGI-5198 has been shown to have anti-tumor efficacy in the TS603 glioma cell line and to lower tumor 2?HG production in HT1080 and U87MG cells with IC50 values of 0.48 and 0.07 μM, respectively. In R132H-IDH1 glioma xenografts, AGI-5198 (450 mg/kg/day) caused 50-60% growth inhibition over a treatment period of three weeks with no affect in the growth of IDH1 wild-type glioma xenografts. Under conditions of near complete 2-HG inhibition, AGI-5198 can induce demethylation of histone H3K9me3 and expression of genes associated with gliogenic differentiation.