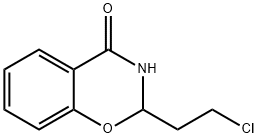
Хлортеноксазин
- английское имяchlorthenoxazine
- CAS №132-89-8
- CBNumberCB9899858
- ФормулаC12H8ClNO
- мольный вес217.65102
- EINECS205-082-3
- номер MDLMFCD00012095
- файл Mol132-89-8.mol
химическое свойство
| Температура плавления | 146-147° (dec) |
| Температура кипения | 303°C (rough estimate) |
| плотность | 1.2414 (rough estimate) |
| показатель преломления | 1.5500 (estimate) |
| температура хранения | Store at -20°C |
| растворимость | Soluble in DMSO |
| пка | 12.70±0.40(Predicted) |
| FDA UNII | KA0B657LV3 |
Хлортеноксазин химические свойства, назначение, производство
Создатель
Reugaril ,Farber ,Italy ,1966Производственный процесс
A mixture of 4 liters chloroform and 1,050 cc ethanol was saturated with dry hydrogen chloride gas at -5°C to +5°C in a vessel having a net volume of 15 liters and provided with a stirring device, reflux cooler, gas feed line, thermometer and dropping funnel. 455 g acrolein which had been precooled to 0°C were added dropwise to the solution over a period of 1 to 2 hours while maintaining the temperature below +5°C and vigorously stirring. 1,070 g salicylamide and 1,080 g glacial acetic acid were added to the resulting solution of beta-chloropropionaldehyde acetal, thereby forming a suspension which was heated to 60°C while stirring. A clear solution was formed which was maintained at 60°C for an additional hour. The solution was allowed to cool to about 40°C and was then washed with water by passing a strong stream of water under the surface of the chloroform and continuously withdrawing the upper phase. When the water had reached a pH of 3-4, the precipitated reaction product was separated by vacuum filtration. The chloroform phase of the filtrate was evaporated under a weak vacuum and the residue was combined with the precipitate first obtained. The combined products were stirred with 2 liters of a 5% sodium hydroxide solution. The raw reaction product was then washed with water, dried and recrystallized from ethanol. The product had the melting point of 146°C to 147°C (decomposition). The yield was 1,260 g, corresponding to 76% of the theoretical yield.Терапевтическая функция
Antipyretic, AnalgesicХлортеноксазин запасные части и сырье
сырьё
Хлортеноксазин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| 025-66113011 18112977050 |
China | 15494 | 58 | |
| 0512-65816829 18662214788 |
China | 9712 | 58 | |
| 888-4366503 | China | 5586 | 58 | |
| 0519-85788828 13775037613 |
China | 3600 | 58 | |
| 13564747822 | China | 952 | 58 | |
| 0519-85524369 | China | 8617 | 58 | |
| 010-60605840 15801484223; |
China | 29774 | 58 | |
| 029-81120477 17792793610 |
China | 10010 | 58 |