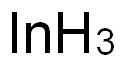
Индий
- английское имяIndium
- CAS №7440-74-6
- CBNumberCB9854194
- ФормулаIn
- мольный вес114.82
- EINECS231-180-0
- номер MDLMFCD00134048
- файл Mol7440-74-6.mol
химическое свойство
| Температура плавления | 156.6 °C (lit.) |
| Температура кипения | 2000 °C |
| плотность | 7.3 g/mL at 25 °C (lit.) |
| давление пара | <0.01 mm Hg ( 25 °C) |
| Fp | 2072°C |
| температура хранения | no restrictions. |
| растворимость | soluble in acid solutions |
| форма | wire |
| цвет | White |
| Удельный вес | 7.31 |
| Flame Color | Indigo blue |
| удельное сопротивление | 8.37 μΩ-cm |
| Растворимость в воде | insoluble |
| Мерк | 14,4947 |
| Пределы воздействия | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Стабильность | Stable. Incompatible with strong acids, strong oxidizing agents, sulfur. |
| LogP | 5.9 at 22℃ |
| Справочник по базе данных CAS | 7440-74-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 045A6V3VFX |
| Справочник по химии NIST | Indium(7440-74-6) |
| Система регистрации веществ EPA | Indium (7440-74-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
больше
| Коды опасности | C,Xn,F,Xi | |||||||||
| Заявления о рисках | 25-26-34-36/37/38-20/21/22-20-11-36/38 | |||||||||
| Заявления о безопасности | 9-16-36/37/39-36-26-45-28 | |||||||||
| РИДАДР | UN 3089 4.1/PG 2 | |||||||||
| OEB | D | |||||||||
| OEL | TWA: 0.1 mg/m3 [*Note: The REL also applies to other indium compounds (as In).] | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | NL1050000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 8112 99 70 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 7440-74-6(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Индий химические свойства, назначение, производство
Описание
Indium lies in Group 13 (13th vertical column of the periodic table). it shows a wide variety of properties. It is considered to be metal of the 'poor metals' group.
Indium (symbol Ga; CAS# 7440-74-6) is widely used in the electronics industry, its radioisotopes are used for diagnostic imaging in medicine. Though this element is not essential for human nutrition, it is widely distributed in low concentrations in the environment (Smith et al., 1978).
Radioisotopes of indium have been used to label phagocytes and lymphocytes to localize inflammatory lesions (Dudley and Marrer, 1952; Abrams and Murrer, 1993). Despite early optimism, neither element has found wide use as a treatment for malignancies.
Химические свойства
Indium is a rare, lustrous silver-white metal with atomic number 49 and In as its atomic symbol. It is widely distributed in the Earth’s crust in minute quantities (0.1 ppm) and is also found in the hydrosphere. Indium belongs to group IIIA in the periodic table. It was found and spectroscopically identified as a minor component in zinc ores and isolated in 1863 by Ferdinand Reich and Theodore Richter. Indium is so named for the indigo blue color that its salts lend to flames. Indium resembles tin in its physical and chemical properties and to some extent in its toxicological properties. It is extremely soft and malleable, with a Brinell test hardness of less than 1 and a Mohs scale hardness of 1.2. In the electromotive series, it appears between iron and tin and does not decompose inwater at boiling temperature. It is stable in air, but when heated, it burns with a nonluminous, blue-red flame yielding indium oxide. The surface of indium remains bright up to its melting point; above this, it forms an oxide film.Indium is flammable in the form of dust, yielding indium oxide when exposed to heat or flame. Mixtures of indium with sulfur ignite when heated. Indium reacts explosively with dinitrogen tetroxide plus acetonitrile. Indium exhibits a violent reaction with mercury(II) bromide at 350°C. Indium is unaffected by water, is attacked by mineral acids, and is very resistant to alkalies.
Физические свойства
Indium is silvery-white and malleable and looks much like aluminum and tin. However,it is softer than lead. Indium metal is so soft that it cannot be “wiped” onto other surfaces aswith a graphite pencil. Because it is noncorrosive and does not oxidize at room temperatures,it can be polished and will hold its shine better than silver. Its melting point is 156.60°C, itsboiling point is 2,075°C, and its density is 7.31 g/cm3.Изотопы
There are a total of 73 isotopes of indium. All are radioactive with relativelyshort half-lives, except two that are considered stable. Isotope In-113 makes up just4.29% of the total indium found in the Earth’s crust. The isotope In-115, with a half-lifeof 4.41×10-14 years contributes the balance (95.71%) of the element’s existence in theEarth’s crust.Происхождение имени
Indium’s name is derived from the Latin word indicum, meaning “indigo,” which is the color of its spectral line when viewed by a spectroscope.Вхождение
Indium is a rather rare metal. It is the 69th most abundant element, which is about asabundant as silver at 0.05 ppm. Although it is widely spread over the Earth’s crust, it is foundin very small concentrations and always combined with other metal ores. It is never found inits natural metallic state.Indium is recovered as a by-product of smelting other metal ores such as aluminum,antimony, cadmium, arsenic, and zinc. About 1,000 kg of indium is recovered each year (ora concentration of 1 part indium per 1000 parts of dust) from the flue stacks (chimneys) ofzinc refineries.
Indium is found in metal ores and minerals located in Russia, Japan, Europe, Peru, andCanada, as well as in the western part of the United States.
Характеристики
Indium has one odd characteristic in that in the form of a sheet, like the metal tin, it willemit a shrieking sound when bent rapidly. Indium has some of the characteristics of othermetals near it in the periodic table and may be thought of as an “extension” of the secondseries of the transition elements. Although it is corrosion-resistant at room temperature, it willoxidize at higher temperatures. It is soluble in acids, but not in alkalis or hot water.Использование
Indium element is used in the synthesis of therapeutic particles containing metal ions; characterized by the use of unique ligand sets capable of making the metal ion complex soluble in biolgical medi a to induce selective toxicity in diseased cells.Определение
indium: Symbol In. A soft silvery elementbelonging to group 13 (formerlyIIIB) of the periodic table; a.n.49; r.a.m. 114.82; r.d. 7.31 (20°C);m.p. 156.6°C; b.p. 2080±2°C. It occursin zinc blende and some iron oresand is obtained from zinc flue dust intotal quantities of about 40 tonnesper annum. Naturally occurring indiumconsists of 4.23% indium–113(stable) and 95.77% indium–115 (halflife6 × 1014 years). There are a furtherfive short-lived radioisotopes.The uses of the metal are small –some special-purpose electroplatesand some special fusible alloys. Severalsemiconductor compounds areused, such as InAs, InP, and InSb.With only three electrons in its valencyshell, indium is an electron acceptorand is used to dope puregermanium and silicon; it forms stableindium(I), indium(II), and indium(III) compounds. The elementwas discovered in 1863 by FerdinandReich (1799–1882) and HieronymusRichter (1824–90).Методы производства
Mineral sources are most commonly dark sphalerite (ZnS), marmatite, and christophite (FeS:ZnS). Indium also occurs in small quantities in tin ores, siderite, and manganese and tungsten ores.Gallium is often associated with indium in zinc and tin ores. Many sulfide ores of copper, iron, lead, cobalt, and bismuth contain small quantities of indium. Zinc smelter flue dusts, in some cases, contain more than 1% indium, and are the largest commercial source of the metal. Other commercial sources are plant residues and dross from the refining of zinc, lead, and cadmium. Indium is recovered from zinc processing residues by acid leaching followed by chemical separation from the accompanying elemental impurities such as zinc, cadmium, aluminum, arsenic, and antimony. Final purification by aqueous electrolysis of the salts at a controlled potential yields a product of 99.9% purity. Canada and Peru supply the greatest amounts of unwrought waste and scrap. Next in order are Japan, Germany, and the United Kingdom. The pattern of indium usage, and potential industrial hazard, is 30% in solders, low-melting alloys, and coatings; 30% in instrument applications and holding devices; 18% in electronic components; 6% in nuclear reactor controls; and 16% in research and other uses.Общее описание
Soft, ductile, shiny, silver-white metal. Mp: 155.6°C; bp: 2080°C. Density 7.31 g cm-3.Профиль реактивности
Indium is a non-combustible solid in bulk form but is flammable in the form of a dust. Reacts with strong oxidizing agents. Reacts explosively with dinitrogen tetraoxide dissolved in acetonitrile. Reacts violently with mercury(II)bromide at 350°C. Mixtures with sulfur ignite when heated.Опасность
Indium metal dust, particles, and vapors are toxic if ingested or inhaled, as are most of thecompounds of indium. This requires the semiconductor and electronics industries that useindium compounds to provide protection for their workers.Угроза здоровью
Indium (In) and compounds cause injury to the lungs, liver and kidneys in animals.There are no reports of toxicity in humans. When indium was applied to the skin there was no evidence of irritation.
Промышленное использование
Indium (symbol In) is a silvery-white metal with a bluish hue, whiter than tin.It is very ductile and does not work-harden, because its recrystallization point is below normal room temperature, and it softens during rolling. The metal is not easily oxidized, but above its melting point, 157 C, it oxidizes and burns with a violet flame.Indium is now obtained as a by-product from a variety of ores. Because of its bright color, light reflectance, and corrosion resistance, it is valued as a plating metal, especially for reflectors. It is softer than lead, but a hard surface is obtained by heating the plated part to diffuse the indium into the base metal. It has high adhesion to other metals. When added to chromium plating baths it reduces brittleness of the chromium.
The three largest uses of indium are in semiconductordevices, bearings, and low meltingpointalloys.
Канцерогенность
Indium has not been tested for its ability to cause cancer in animals. However, the probable carcinogenic properties of indium are linked to alterations in the synthesis and maintenance of enzyme systems that metabolize organic carcinogens. A compromise in the ability of these metabolic systems would lead to altered cellular responses to organic carcinogenic substances.Методы очистки
Before use, the metal surface is cleaned with dilute HNO3, followed by thorough washing with water and an alcohol rinse. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 856 1963.]Структура и конформация
The space lattice of Indium belongs to the tetragonal system and its deformed face centered cubic D4h (4 atoms within a unit cell) has lattice constants of a=0.4588 nm, c=0.4938 nm, and In–In=0.324 nm.Индий запасные части и сырье
запасной предмет
Индий поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86 319 5273535 | CHINA | 287 | 58 |