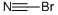
Цианоген бромид
- английское имяCyanogen bromide
- CAS №506-68-3
- CBNumberCB9853692
- ФормулаCBrN
- мольный вес105.92
- EINECS208-051-2
- номер MDLMFCD00011597
- файл Mol506-68-3.mol
| Температура плавления | 50-53 °C (lit.) |
| Температура кипения | 61-62 °C (lit.) |
| плотность | 1.443 g/mL at 25 °C |
| плотность пара | 3.65 (vs air) |
| давление пара | 100 mm Hg ( 22.6 °C) |
| показатель преломления | 1.4670 (estimate) |
| Fp | 61.4°C |
| температура хранения | 2-8°C |
| растворимость | Soluble in chloroform, dichloromethane, ethanol, diethyl ether, benzene and acetonitrile. |
| форма | Solution |
| цвет | White |
| Запах | Penetrating odor |
| Растворимость в воде | decomposed slowly by cold H2O [HAW93] |
| Чувствительный | Moisture & Light Sensitive |
| Мерк | 14,2693 |
| БРН | 1697296 |
| Пределы воздействия | No exposure limit is set. However, on the basis of the exposure limits of related compounds a ceiling limit of 0.5 ppm (2 mg/m3) is recommended. |
| Стабильность | Stable. Reacts violently with water and with mineral and organic acids. |
| Справочник по базе данных CAS | 506-68-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | OS382OHJ8P |
| Справочник по химии NIST | Cyanogen bromide(506-68-3) |
| Система регистрации веществ EPA | Cyanogen bromide (506-68-3) |
| UNSPSC Code | 12352117 |
| NACRES | NA.22 |
| Коды опасности | T+,N,F,C | |||||||||
| Заявления о рисках | 26/27/28-34-50/53-40-11-36/37-32-51/53 | |||||||||
| Заявления о безопасности | 53-28-36/37/39-45-60-61-26-16-7/9-29-7 | |||||||||
| РИДАДР | UN 3390 6.1/PG 1 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GT2100000 | |||||||||
| F | 8-17-19-21 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 28530090 | |||||||||
| Банк данных об опасных веществах | 506-68-3(Hazardous Substances Data) | |||||||||
| Токсичность | LCLO inhal (human) 92 ppm (398 mg/m3; 10 min) LCLO inhal (mouse) 115 ppm (500 mg/m3; 10 min) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H400:Чрезвычайно токсично для водных организмов.
H300+H310+H330:Смертельно при проглатывании, при контакте с кожей или при вдыхании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Цианоген бромид химические свойства, назначение, производство
Химические свойства
Cyanogen bromide is a colorless or white, volatile, crystalline solid with a penetrating odor.Использование
Cyanogen bromide is used in organic synthesis and as a reagent in bioanalysis.Методы производства
Cyanogen bromide may be prepared by either the action of bromine on potassium cyanide or the interaction of sodium bromide, sodium cyanide, sodium chlorate, and sulfuric acid.Общее описание
Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. Cyanogen bromide is slightly soluble in water. Cyanogen bromide is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a flammable and poisonous gas. Contamination with many materials can cause rapid decomposition of the material. Cyanogen bromide is toxic by inhalation of its vapors or by the hydrogen cyanide from decomposition or by ingestion. Toxic oxides of nitrogen are produced in fire involving Cyanogen bromide. Cyanogen bromide is used in gold extraction, to make other chemicals, and as a fumigant.Реакции воздуха и воды
Cyanogen bromide is slightly soluble in water. Cyanogen bromide is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a poison gas.Профиль реактивности
Cyanogen bromide is not combustible itself, but impure Cyanogen bromide decomposes rapidly and tends to explode. A violent reaction may take place on contact with large quantities of acid. Avoid physical damage, contact with acids or water, and store away from a location where water may be needed for fire control. [EPA, 1998]. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).Угроза здоровью
Exposure to cyanogen bromide is dangerous. The chemical substance is poisonous and causes fatal injury if swallowed, inhaled, or absorbed through the skin. It is corrosive and the vapors cause severe irritation to the eyes and respiratory tract, and cause burns to any area of contact. On contact with acids, cyanogen bromide liberates poisonous gas, affecting the blood, cardiovascular system, CNS, and thyroid.Пожароопасность
Cyanogen bromide is not combustible itself, but impure Cyanogen bromide decomposes rapidly and tends to explode. A violent reaction may take place on contact with large quantities of acid. Vapors are highly irritating. When material is heated to decomposition, Cyanogen bromide emits very toxic fumes of cyanide and bromide. Avoid water, acids. Avoid physical damage, contact with acids or water, and store away from a location where water may be needed for fire control.Воспламеняемость и взрывоопасность
Cyanogen bromide is noncombustible. Impure material decomposes rapidly and can be explosive.Профиль безопасности
A human and experimental poison by inhalation. Corrosive. When heated to decomposition it emits very toxic fumes of CNand Br-. Possibly unstable. See also other cyanogen entries; CYANIDE; and BROMIDES.Возможный контакт
Used as an activating reagent for insoluble supports for affinity absorption. In danger are those manufacturing this compound or using it in organic synthesis or as a fumigant; in textile treatment; in gold cyaniding. It may have been used as a military poison gas.хранилище
work with BrCN should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Containers of cyanogen bromide should be kept tightly sealed and stored under nitrogen in a secondary container in a refrigerator.Перевозки
UN1889 Cyanogen bromide, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. A DOT regulated marine pollutant.Методы очистки
All operations with this substance should be performed in a very efficient fume cupboard-it is very POISONOUS and should be handled in small amounts. Fresh commercial material is satisfactory for nearly all purposes and does not need to be purified. It is a white crystalline solid with a strong cyanide odour. If it is reddish in colour and partly liquid or paste-like, then it is too far gone to be purified, and fresh material should be sought. It can be purified by distillation using small amounts at a time, and using a short wide-bore condenser because it readily solidifies to a crystalline white solid which may clog the condenser. An appropriate gas mask should be used when transferring the molten solid from one container to another, and the operation should be done in an efficient fume cupboard. The melting point (m 49-51o) should be measured in a sealed tube. [Hartman & Dreger Org Synth Coll Vol II 150 1948.]Несовместимости
May be unstable unless dry and pure; impure cyanogen bromide decomposes rapidly and tends to explode. Cyanogen bromide decomposes violently on heat- ing or on contact with water, acids, or acid vapors; produc- ing highly toxic and flammable hydrogen cyanide and corrosive hydrogen bromide. Avoid physical damage, con- tact with acids or water, and store away from a location where water may be needed for fire control . Violent reaction with ammonia, amines.Утилизация отходов
May be added to strong alka- line solution of calcium hypochlorite, let stand for 24 hours and flush to sewer. May also be dissolved in flammable solvent and sprayed into an incinerator equipped with after- burner and scrubber.Цианоген бромид запасные части и сырье
сырьё
запасной предмет
- Диэтилцианамид
- (-)-2-азабицикло[2.2.1]гепт-5-ен-3-он
- Диэтилцианофосфоната
- 4-МЕТОКСИ-2-МОРФОЛИН-4-ИЛ-ТИАЗОЛ-5-КАРБАЛЬДЕГИД
- 1,1,1,3,3,3-гексафтор-2-пропанол
- Флуоксетин
- 2-(4-difluoromethoxy)phenyl-3-methyl butyric acid
- Морфолин-4-карбoтиоамид
- Admire
- 2-бромфуран
1of4
Цианоген бромид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-29-87569266 15319487004 |
China | 3939 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-0519-85551759 +8613506123987 |
China | 8840 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |