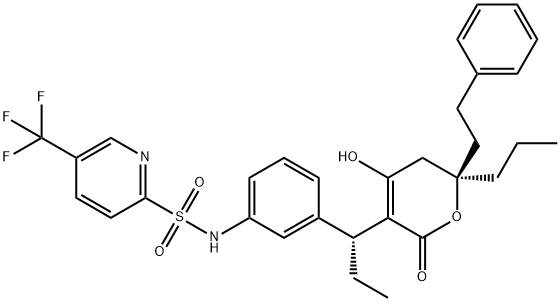
Типранавир
- английское имяTipranavir
- CAS №174484-41-4
- CBNumberCB9501005
- ФормулаC31H33F3N2O5S
- мольный вес602.66
- файл Mol174484-41-4.mol
химическое свойство
| Температура плавления | 86-890C |
| альфа | D +20° (ethanol) |
| Температура кипения | 712.3±70.0 °C(Predicted) |
| плотность | 1.313±0.06 g/cm3(Predicted) |
| температура хранения | -20°C Freezer |
| растворимость | Methanol (Slightly) |
| форма | Solid |
| пка | 4.50±1.00(Predicted) |
| цвет | White to Pale Yellow |
| BCS Class | 2 |
| Словарь онкологических терминов NCI | Aptivus |
| FDA UNII | ZZT404XD09 |
| Код УВД | J05AE09 |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
| Банк данных об опасных веществах | 174484-41-4(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
вредная бумага
H411:Токсично для водных организмов с долгосрочными последствиями.
Типранавир химические свойства, назначение, производство
Химические свойства
White SolidОпределение
ChEBI: A pyridine-2-sulfonamide substituted at C-5 by a trifluoromethyl group and at the sulfonamide nitrogen by a dihydropyrone-containing m-tolyl substituent. It is an HIV-1 protease inhibitor.Приобретенная устойчивость
In a study of 105 viruses resistant to other protease inhibitors, 90% exhibited a more than four-fold decrease in susceptibility and 2% high-level resistance (>10-fold decrease). The predominant emerging mutations in use with ritonavir are L33F/I/V, V82T/L and I84V. Combination of all three of these mutations is usually required for reduced susceptibility. Mutations at positions 47, 58 and 74 are also associated with resistance.Общее описание
Tipranavir is unique among the PIs because it is not a peptidomimeticcompound. It does appear to bind to the activesite of HIV-1 protease the same as the peptidomimetics do.The benefit of this agent is that, because it is a differentchemical structure, cross-resistance does not develop tothe same extent as seen with the peptidomimetics. Thedrug is administered with a booster dose of ritonavir. Thisprotocol inhibits CYP3A4, causing the levels of tipranavirto increase.Фармацевтические приложения
A non-peptidic protease inhibitor formulated as capsules or solution for oral use.Механизм действия
Tipranavir appears to be bound to the same active site of HIV-1 protease as the peptidomimetics are, but because of its different chemical structure, cross-resistance is significantly less than that seen between the peptidomimetics. The drug suppresses viral replication in various strains of HIV-1 in vitro, and when combined with azothymidine or delaviridine, synergistic activity is noted in vitro. Tipranavir has an advantage over the other PIs in that it is not as strongly bound to plasma protein as the earlier PIs are, a property that reduces the 90% inhibition concentration.Фармакокине?тика
Oral absorption: Not known/availableCmax 500 mg + 200 mg ritonavir twice: c. 57.2 mg/L (female);
daily: 46.8 mg/L (male)
Cmin 500 mg + 200 mg ritonavir twice: c. 25.1 mg/L (female);
daily: 21.5 mg/L (male)
Plasma half-life: c. 5.5 h (female); 6 h (male)
Volume of distribution: Not known/available
Plasma protein binding: >99.9%
Absorption and distribution
The combination with ritonavir may be taken with or without food. No studies have been conducted to determine the distribution into human CSF, semen or breast milk.
Metabolism and excretion
Metabolism in the presence of 200 mg ritonavir is minimal. Around 82% is excreted in the feces and 4% in the urine. In mild hepatic impairment it should be used with caution; it should not be used in moderate or severe hepatic impairment.
Клиническое использование
Treatment (in combination with other antiretroviral drugs) of HIV-1 infection in patients unresponsive to more than one other protease inhibitorПобочные эффекты
Adverse effects include nausea, vomiting, diarrhea, fatigue and headache. In studies of ritonavir-boosted regimens higher rates of hepatotoxicity have been observed with tipranavir than with other protease inhibitors. In addition, 14 reports of intracranial bleeding (eight fatal cases) associated with tipranavir have been reported. It has been associated with dyslipidemia to a greater extent than other protease inhibitors.Типранавир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615350851019 | China | 1001 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 0917-3909592 13892490616 |
China | 9310 | 58 | |
| +8615056975894 | CHINA | 9911 | 58 | |
| +1-2135480471 +1-2135480471 |
China | 10473 | 58 | |
| +86-27-50766799 +8618062016861 |
China | 19987 | 58 |