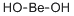
Бериллия гидроксид
- английское имяBERYLLIUM HYDROXIDE
- CAS №13327-32-7
- CBNumberCB9449796
- ФормулаBeH2O2
- мольный вес43.03
- EINECS236-368-6
- номер MDLMFCD00049856
- файл Mol13327-32-7.mol
химическое свойство
| Температура плавления | 105 °C (decomp) |
| плотность | 1.92 |
| растворимость | slightly soluble in H2O, alkaline solutions; soluble in acid solutions |
| форма | amorphous powder or crystals |
| цвет | Colorless, orthorhombic, amorphous powder orcrystals |
| РН | 7.9(1 mM solution);7.9(10 mM solution);7.9(100 mM solution) |
| Растворимость в воде | very slightly soluble H2O, dilute alkali; soluble hot NaOH, acids [MER06] |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | 2X0LRF1T6Q |
| Система регистрации веществ EPA | Beryllium hydroxide (Be(OH)2) (13327-32-7) |
| РИДАДР | 1566 |
| Класс опасности | 6.1(a) |
| Группа упаковки | II |
| Банк данных об опасных веществах | 13327-32-7(Hazardous Substances Data) |
| Токсичность | LDLo ivn-rat: 3821 mg/kg XEURAQ UR-70,1949 |
Бериллия гидроксид химические свойства, назначение, производство
Химические свойства
White powder. Decomposes to the oxide at 138C. Soluble in acids and alkalies; insoluble in water.Физические свойства
Beryllium Hydroxide can be prepared by reacting the oxide with water:BeO+H2O→Be(OH)2
If heated, it decomposes at 1273°C. Its solubility in water is slight and its solubility product Ksp(H2O) is 6.92×10-22. It is soluble in acids and bases.
Beryllium hydroxide exists in three forms: as a metastable tetragonal crystalline solid (a-Be(OH)2; as a stable orthorhombic crystalline solid (b-Be(OH)2); and in a slightly basic pH, it appears as a slimy, gelatinous substance (Be(OH)42-).
Использование
Beryllium hydroxide is a strategic component concerning the usage of beryllium metal and its compounds in industry. Beryllium hydroxide is the key compound used to prepare a variety of beryllium products.Note that Be(OH)2 is the primary product used to prepare the metal and various beryllium salts.The largest usage of beryllium hydroxide is to make the oxide or the metal that is used in many industries. The total tonnage is not large but substantial. Beryllium hydroxide is available commercially in large quantities.
Определение
beryllium hydroxide: A whitecrystalline compound, Be(OH)2, precipitatedfrom solutions of berylliumsalts by adding alkali. Like the oxide,it is amphoteric and dissolves in excessalkali to give beryllates.Подготовка
Beryllium hydroxide, Be(OH)2, is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially it is produced as a by-product in the extraction of beryllium metal from the ores, “beryl” and “bertrandite” When alkali is added to beryllium salt solutions, the a-form (a gel) is formed. If this left to stand or boiled the rhombic b-form precipitates. This has the Zn(OH)2 structure with tetrahedral beryllium centers. The alkali dissolution forms a “beryllate”:2NaOH(aq)+Be(OH)2(solid) → Na2Be(OH)4(aq)
With acids, beryllium salts are formed involving the Be(OH)+ cation. With sulfuric acid, H2SO4:
2Be(OH)2+H2SO4→[Be(OH)]2SO4+ 2H2O
Thus, the probable formula for beryllium hydroxide is Be(OH)OH. However, this has never been reported. Beryllium hydroxide dehydrates completely at 400°C to form a white powder of acid soluble beryllium oxide:
Be(OH)2+ heat → BeO+H2O
Further heating at higher temperature produces acid insoluble BeO.
Опасность
Very toxic.Профиль безопасности
Confirmed carcinogen withexperimental carcinogenic and tumorigenic data. Poisonby intravenous route. When heated to decomposition it emitsvery toxic fumes of BeO.Бериллия гидроксид запасные части и сырье
сырьё
Бериллия гидроксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 0731-85118349 18874718518 |
China | 1595 | 50 | |
| 029-87576359 15353716720 |
China | 10009 | 58 |