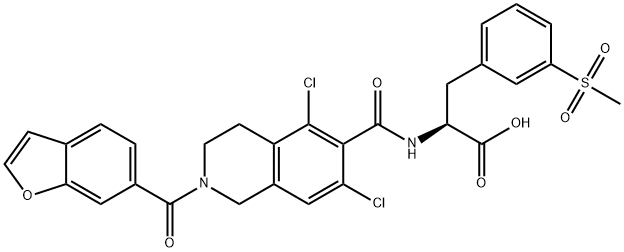
Лифитеграст
- английское имяlifitegrast
- CAS №1025967-78-5
- CBNumberCB92738258
- ФормулаC29H24Cl2N2O7S
- мольный вес615.48
- EINECS813-044-8
- номер MDLMFCD28502439
- файл Mol1025967-78-5.mol
химическое свойство
| Температура плавления | >163°C (dec.) |
| Температура кипения | 811.9±65.0 °C(Predicted) |
| плотность | 1.479±0.06 g/cm3(Predicted) |
| температура хранения | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| растворимость | DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Sonicated) |
| форма | Solid |
| пка | 3.14±0.10(Predicted) |
| цвет | White to Off-White |
| Стабильность | Hygroscopic |
| ИнЧИКей | JFOZKMSJYSPYLN-QHCPKHFHSA-N |
| SMILES | C(O)(=O)[C@H](CC1=CC=CC(S(C)(=O)=O)=C1)NC(C1C(Cl)=CC2=C(C=1Cl)CCN(C(C1C=C3OC=CC3=CC=1)=O)C2)=O |
| FDA UNII | 038E5L962W |
| Код УВД | S01XA25 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Лифитеграст химические свойства, назначение, производство
Описание
Lifitegrast (Xiidra; lifitegrast sodium; SAR1118; SHP606; SPD606) is a lymphocyte functionassociated antigen-1 (LFA-1) antagonist. It inhibits T-cell inflammation by blocking the binding of two key surface proteins (LFA-1 and ICAM-1) that mediate the chronic inflammatory cascade associated with dry eye disease. In a phase III clinical trial, lifitegrast 5% ophthalmic solution (50 mg/mL) is administered as a single 0.2mL eye drop twice a day into each eye for an 84 day treatment period.
Lifitegrast does not currently have Marketing Authorisation in the EU for any indication. Lifitegrast is licensed for use in the USA for treatment of the signs and symptoms of dry eye disease. The most common adverse reactions (incidence 5-25%) reported following the use of lifitegrast are instillation site irritation, dysgeusia, and decreased visual acuity.
Химические свойства
Lifitegrast is a white to off-white powder which is soluble in water.Использование
Lifitegrast is used for the treatment of signs and symptons of dry eye diseases. It also Inhibites corneal inflammation that is capable of causing pains, blurred vision and ocular discomfort in sufferer.Показания
Topical lifitegrast was approved by the FDA for the treatment of dry eye. Lifitegrast decreases inflammation by blocking the interaction between intercellular adhesion molcule 1 and lymphocyte function- -associated antigen 1. In four, large, multicellular, randomized clinical trials, lifitegrast was shown to be effective in improving the signs and symptoms of dry eye. The side effects of lifitegrast include transient ocular irritation and dysgeusia. Further studies are needed to explore the effectiveness of combination therapy such as the concomitant use of topical cyclosporine and topical liftegrast.Определение
ChEBI: An N-acyl-L-alpha-amino acid obtained by formal condensation of the carboxy group of N-[2-(1-benzofuran-6-carbonyl)]-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid with he amino group of 3-(methanesulfonyl)-L-phenylalanine. Used for treatment of keratoconjunctivitis sicca (dry eye syndrome).Механизм действия
Lifitegrast binds to the integrin lymphocyte function-associated antigen-1 (LFA-1), a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular adhesion molecule-1 (ICAM-1). ICAM-1 may be overexpressed in corneal and conjunctival tissues in dry eye disease. LFA-1/ICAM-1 interaction can contribute to the formation of an immunological synapse resulting in T-cell activation and migration to target tissues. In vitro studies demonstrated that lifitegrast may inhibit T-cell adhesion to ICAM-1 in a human T-cell line and may inhibit secretion of inflammatory cytokines in human peripheral blood mononuclear cells. The exact mechanism of action of lifitegrast in dry eye disease is not known.Фармакокине?тика
In a subset of dry eye disease patients (n=47) enrolled in a Phase 3 trial, the pre-dose (trough) plasma concentrations of lifitegrast were measured after 180 and 360 days of topical ocular dosing (1 drop twice daily) with Xiidra (lifitegrast ophthalmic solution) 5%. A total of nine (9) of the 47 patients (19%) had plasma lifitegrast trough concentrations above 0.5 ng/mL (the lower limit of assay quantitation). Trough plasma concentrations that could be quantitated ranged from 0.55 ng/mL to 3.74 ng/mL.Побочные эффекты
A multicenter, randomized, double-masked, placebo-controlled phase 3 study (n = 331) evaluating the safety of lifitegrast ophthalmic solution for the treatment of dry eye disease reported the most common non-ocular effect was dysgeusia (change in taste) occurring in 16.4% of patients in the lifitegrast group and 1.8% of the placebo group.использованная литература
[1] Stacy L. Haber. “Lifitegrast: a novel drug for patients with dry eye disease.” ACS Applied Bio Materials (2019).Лифитеграст запасные части и сырье
сырьё
- 5,7-дихлор-2-тритил-1,2,3,4-тетрагидроизохинолин-6-карбоновая кислота
- benzyl (S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoate
- (S)-2-(трет-бутоксикарбониламино)-3-(3-(метилсульфонил)фенил)пропановая кислота
- 3,5-Dichlorobenzaldehyde
- lifitegrast impurity C
1of2
Лифитеграст поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-021-38228826 +86-13916602830 |
China | 140 | 58 | ||
| +16313385890 | United States | 3868 | 58 | ||
| +86-53169958659 +86-13153181156 |
China | 294 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 |